Oestradiol (quantile) - Whole_Blood
wesleycrouse
2021-09-09
Last updated: 2021-09-09
Checks: 6 1
Knit directory: ctwas_applied/
This reproducible R Markdown analysis was created with workflowr (version 1.6.2). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
The R Markdown file has unstaged changes. To know which version of the R Markdown file created these results, you’ll want to first commit it to the Git repo. If you’re still working on the analysis, you can ignore this warning. When you’re finished, you can run wflow_publish to commit the R Markdown file and build the HTML.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20210726) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version 59e5f4d. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Unstaged changes:
Modified: analysis/ukb-d-30500_irnt_Liver.Rmd
Modified: analysis/ukb-d-30500_irnt_Whole_Blood.Rmd
Modified: analysis/ukb-d-30600_irnt_Liver.Rmd
Modified: analysis/ukb-d-30600_irnt_Whole_Blood.Rmd
Modified: analysis/ukb-d-30610_irnt_Liver.Rmd
Modified: analysis/ukb-d-30610_irnt_Whole_Blood.Rmd
Modified: analysis/ukb-d-30620_irnt_Liver.Rmd
Modified: analysis/ukb-d-30620_irnt_Whole_Blood.Rmd
Modified: analysis/ukb-d-30630_irnt_Liver.Rmd
Modified: analysis/ukb-d-30630_irnt_Whole_Blood.Rmd
Modified: analysis/ukb-d-30640_irnt_Liver.Rmd
Modified: analysis/ukb-d-30640_irnt_Whole_Blood.Rmd
Modified: analysis/ukb-d-30650_irnt_Liver.Rmd
Modified: analysis/ukb-d-30650_irnt_Whole_Blood.Rmd
Modified: analysis/ukb-d-30660_irnt_Liver.Rmd
Modified: analysis/ukb-d-30660_irnt_Whole_Blood.Rmd
Modified: analysis/ukb-d-30670_irnt_Liver.Rmd
Modified: analysis/ukb-d-30670_irnt_Whole_Blood.Rmd
Modified: analysis/ukb-d-30680_irnt_Liver.Rmd
Modified: analysis/ukb-d-30690_irnt_Liver.Rmd
Modified: analysis/ukb-d-30690_irnt_Whole_Blood.Rmd
Modified: analysis/ukb-d-30700_irnt_Liver.Rmd
Modified: analysis/ukb-d-30700_irnt_Whole_Blood.Rmd
Modified: analysis/ukb-d-30710_irnt_Liver.Rmd
Modified: analysis/ukb-d-30710_irnt_Whole_Blood.Rmd
Modified: analysis/ukb-d-30720_irnt_Liver.Rmd
Modified: analysis/ukb-d-30720_irnt_Whole_Blood.Rmd
Modified: analysis/ukb-d-30730_irnt_Liver.Rmd
Modified: analysis/ukb-d-30740_irnt_Liver.Rmd
Modified: analysis/ukb-d-30740_irnt_Whole_Blood.Rmd
Modified: analysis/ukb-d-30750_irnt_Liver.Rmd
Modified: analysis/ukb-d-30750_irnt_Whole_Blood.Rmd
Modified: analysis/ukb-d-30760_irnt_Liver.Rmd
Modified: analysis/ukb-d-30760_irnt_Whole_Blood.Rmd
Modified: analysis/ukb-d-30770_irnt_Liver.Rmd
Modified: analysis/ukb-d-30770_irnt_Whole_Blood.Rmd
Modified: analysis/ukb-d-30780_irnt_Liver.Rmd
Modified: analysis/ukb-d-30780_irnt_Whole_Blood.Rmd
Modified: analysis/ukb-d-30790_irnt_Liver.Rmd
Modified: analysis/ukb-d-30800_irnt_Liver.Rmd
Modified: analysis/ukb-d-30800_irnt_Whole_Blood.Rmd
Modified: analysis/ukb-d-30810_irnt_Liver.Rmd
Modified: analysis/ukb-d-30820_irnt_Liver.Rmd
Modified: analysis/ukb-d-30830_irnt_Liver.Rmd
Modified: analysis/ukb-d-30840_irnt_Liver.Rmd
Modified: analysis/ukb-d-30850_irnt_Liver.Rmd
Modified: analysis/ukb-d-30860_irnt_Liver.Rmd
Modified: analysis/ukb-d-30870_irnt_Liver.Rmd
Modified: analysis/ukb-d-30880_irnt_Liver.Rmd
Modified: analysis/ukb-d-30890_irnt_Liver.Rmd
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were made to the R Markdown (analysis/ukb-d-30800_irnt_Whole_Blood.Rmd) and HTML (docs/ukb-d-30800_irnt_Whole_Blood.html) files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view the files as they were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | cbf7408 | wesleycrouse | 2021-09-08 | adding enrichment to reports |
| html | cbf7408 | wesleycrouse | 2021-09-08 | adding enrichment to reports |
| Rmd | 4970e3e | wesleycrouse | 2021-09-08 | updating reports |
| html | 4970e3e | wesleycrouse | 2021-09-08 | updating reports |
| Rmd | dfd2b5f | wesleycrouse | 2021-09-07 | regenerating reports |
| html | dfd2b5f | wesleycrouse | 2021-09-07 | regenerating reports |
| Rmd | 61b53b3 | wesleycrouse | 2021-09-06 | updated PVE calculation |
| html | 61b53b3 | wesleycrouse | 2021-09-06 | updated PVE calculation |
| Rmd | 837dd01 | wesleycrouse | 2021-09-01 | adding additional fixedsigma report |
| Rmd | d0a5417 | wesleycrouse | 2021-08-30 | adding new reports to the index |
| Rmd | 0922de7 | wesleycrouse | 2021-08-18 | updating all reports with locus plots |
| html | 0922de7 | wesleycrouse | 2021-08-18 | updating all reports with locus plots |
| html | 1c62980 | wesleycrouse | 2021-08-11 | Updating reports |
| Rmd | 5981e80 | wesleycrouse | 2021-08-11 | Adding more reports |
| html | 5981e80 | wesleycrouse | 2021-08-11 | Adding more reports |
Overview
These are the results of a ctwas analysis of the UK Biobank trait Oestradiol (quantile) using Whole_Blood gene weights.
The GWAS was conducted by the Neale Lab, and the biomarker traits we analyzed are discussed here. Summary statistics were obtained from IEU OpenGWAS using GWAS ID: ukb-d-30800_irnt. Results were obtained from from IEU rather than Neale Lab because they are in a standardard format (GWAS VCF). Note that 3 of the 34 biomarker traits were not available from IEU and were excluded from analysis.
The weights are mashr GTEx v8 models on Whole_Blood eQTL obtained from PredictDB. We performed a full harmonization of the variants, including recovering strand ambiguous variants. This procedure is discussed in a separate document. (TO-DO: add report that describes harmonization)
LD matrices were computed from a 10% subset of Neale lab subjects. Subjects were matched using the plate and well information from genotyping. We included only biallelic variants with MAF>0.01 in the original Neale Lab GWAS. (TO-DO: add more details [number of subjects, variants, etc])
Weight QC
TO-DO: add enhanced QC reporting (total number of weights, why each variant was missing for all genes)
qclist_all <- list()
qc_files <- paste0(results_dir, "/", list.files(results_dir, pattern="exprqc.Rd"))
for (i in 1:length(qc_files)){
load(qc_files[i])
chr <- unlist(strsplit(rev(unlist(strsplit(qc_files[i], "_")))[1], "[.]"))[1]
qclist_all[[chr]] <- cbind(do.call(rbind, lapply(qclist,unlist)), as.numeric(substring(chr,4)))
}
qclist_all <- data.frame(do.call(rbind, qclist_all))
colnames(qclist_all)[ncol(qclist_all)] <- "chr"
rm(qclist, wgtlist, z_gene_chr)
#number of imputed weights
nrow(qclist_all)[1] 11095#number of imputed weights by chromosome
table(qclist_all$chr)
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15
1129 747 624 400 479 621 560 383 404 430 682 652 192 362 331
16 17 18 19 20 21 22
551 725 159 911 313 130 310 #proportion of imputed weights without missing variants
mean(qclist_all$nmiss==0)[1] 0.762776Load ctwas results
#load ctwas results
ctwas_res <- data.table::fread(paste0(results_dir, "/", analysis_id, "_ctwas.susieIrss.txt"))
#make unique identifier for regions
ctwas_res$region_tag <- paste(ctwas_res$region_tag1, ctwas_res$region_tag2, sep="_")
#compute PVE for each gene/SNP
ctwas_res$PVE = ctwas_res$susie_pip*ctwas_res$mu2/sample_size #check PVE calculation
#separate gene and SNP results
ctwas_gene_res <- ctwas_res[ctwas_res$type == "gene", ]
ctwas_gene_res <- data.frame(ctwas_gene_res)
ctwas_snp_res <- ctwas_res[ctwas_res$type == "SNP", ]
ctwas_snp_res <- data.frame(ctwas_snp_res)
#add gene information to results
sqlite <- RSQLite::dbDriver("SQLite")
db = RSQLite::dbConnect(sqlite, paste0("/project2/compbio/predictdb/mashr_models/mashr_", weight, ".db"))
query <- function(...) RSQLite::dbGetQuery(db, ...)
gene_info <- query("select gene, genename from extra")
gene_info <- query("select gene, genename, gene_type from extra")
RSQLite::dbDisconnect(db)
ctwas_gene_res <- cbind(ctwas_gene_res, gene_info[sapply(ctwas_gene_res$id, match, gene_info$gene), c("genename", "gene_type")])
#add z score to results
load(paste0(results_dir, "/", analysis_id, "_expr_z_gene.Rd"))
ctwas_gene_res$z <- z_gene[ctwas_gene_res$id,]$z
#load(paste0(results_dir, "/", analysis_id, "_expr_z_snp.Rd")) #for new version, stored after harmonization
z_snp <- readRDS(paste0(results_dir, "/", trait_id, ".RDS")) #for old version, unharmonized
z_snp <- z_snp[z_snp$id %in% ctwas_snp_res$id,] #subset snps to those included in analysis, note some are duplicated, need to match which allele was used
ctwas_snp_res$z <- z_snp$z[match(ctwas_snp_res$id, z_snp$id)] #for duplicated snps, this arbitrarily uses the first allele
ctwas_snp_res$z_flag <- as.numeric(ctwas_snp_res$id %in% z_snp$id[duplicated(z_snp$id)]) #mark the unclear z scores, flag=1
#formatting and rounding for tables
ctwas_gene_res$z <- round(ctwas_gene_res$z,2)
ctwas_snp_res$z <- round(ctwas_snp_res$z,2)
ctwas_gene_res$susie_pip <- round(ctwas_gene_res$susie_pip,3)
ctwas_snp_res$susie_pip <- round(ctwas_snp_res$susie_pip,3)
ctwas_gene_res$mu2 <- round(ctwas_gene_res$mu2,2)
ctwas_snp_res$mu2 <- round(ctwas_snp_res$mu2,2)
ctwas_gene_res$PVE <- signif(ctwas_gene_res$PVE, 2)
ctwas_snp_res$PVE <- signif(ctwas_snp_res$PVE, 2)
#merge gene and snp results with added information
ctwas_gene_res$z_flag=NA
ctwas_snp_res$genename=NA
ctwas_snp_res$gene_type=NA
ctwas_res <- rbind(ctwas_gene_res,
ctwas_snp_res[,colnames(ctwas_gene_res)])
#store columns to report
report_cols <- colnames(ctwas_gene_res)[!(colnames(ctwas_gene_res) %in% c("type", "region_tag1", "region_tag2", "cs_index", "gene_type", "z_flag", "id", "chrom", "pos"))]
first_cols <- c("genename", "region_tag")
report_cols <- c(first_cols, report_cols[!(report_cols %in% first_cols)])
report_cols_snps <- c("id", report_cols[-1])
#get number of SNPs from s1 results; adjust for thin
ctwas_res_s1 <- data.table::fread(paste0(results_dir, "/", analysis_id, "_ctwas.s1.susieIrss.txt"))
n_snps <- sum(ctwas_res_s1$type=="SNP")/thin
rm(ctwas_res_s1)Check convergence of parameters
library(ggplot2)
library(cowplot)
********************************************************Note: As of version 1.0.0, cowplot does not change the default ggplot2 theme anymore. To recover the previous behavior, execute:
theme_set(theme_cowplot())********************************************************load(paste0(results_dir, "/", analysis_id, "_ctwas.s2.susieIrssres.Rd"))
df <- data.frame(niter = rep(1:ncol(group_prior_rec), 2),
value = c(group_prior_rec[1,], group_prior_rec[2,]),
group = rep(c("Gene", "SNP"), each = ncol(group_prior_rec)))
df$group <- as.factor(df$group)
df$value[df$group=="SNP"] <- df$value[df$group=="SNP"]*thin #adjust parameter to account for thin argument
p_pi <- ggplot(df, aes(x=niter, y=value, group=group)) +
geom_line(aes(color=group)) +
geom_point(aes(color=group)) +
xlab("Iteration") + ylab(bquote(pi)) +
ggtitle("Prior mean") +
theme_cowplot()
df <- data.frame(niter = rep(1:ncol(group_prior_var_rec), 2),
value = c(group_prior_var_rec[1,], group_prior_var_rec[2,]),
group = rep(c("Gene", "SNP"), each = ncol(group_prior_var_rec)))
df$group <- as.factor(df$group)
p_sigma2 <- ggplot(df, aes(x=niter, y=value, group=group)) +
geom_line(aes(color=group)) +
geom_point(aes(color=group)) +
xlab("Iteration") + ylab(bquote(sigma^2)) +
ggtitle("Prior variance") +
theme_cowplot()
plot_grid(p_pi, p_sigma2)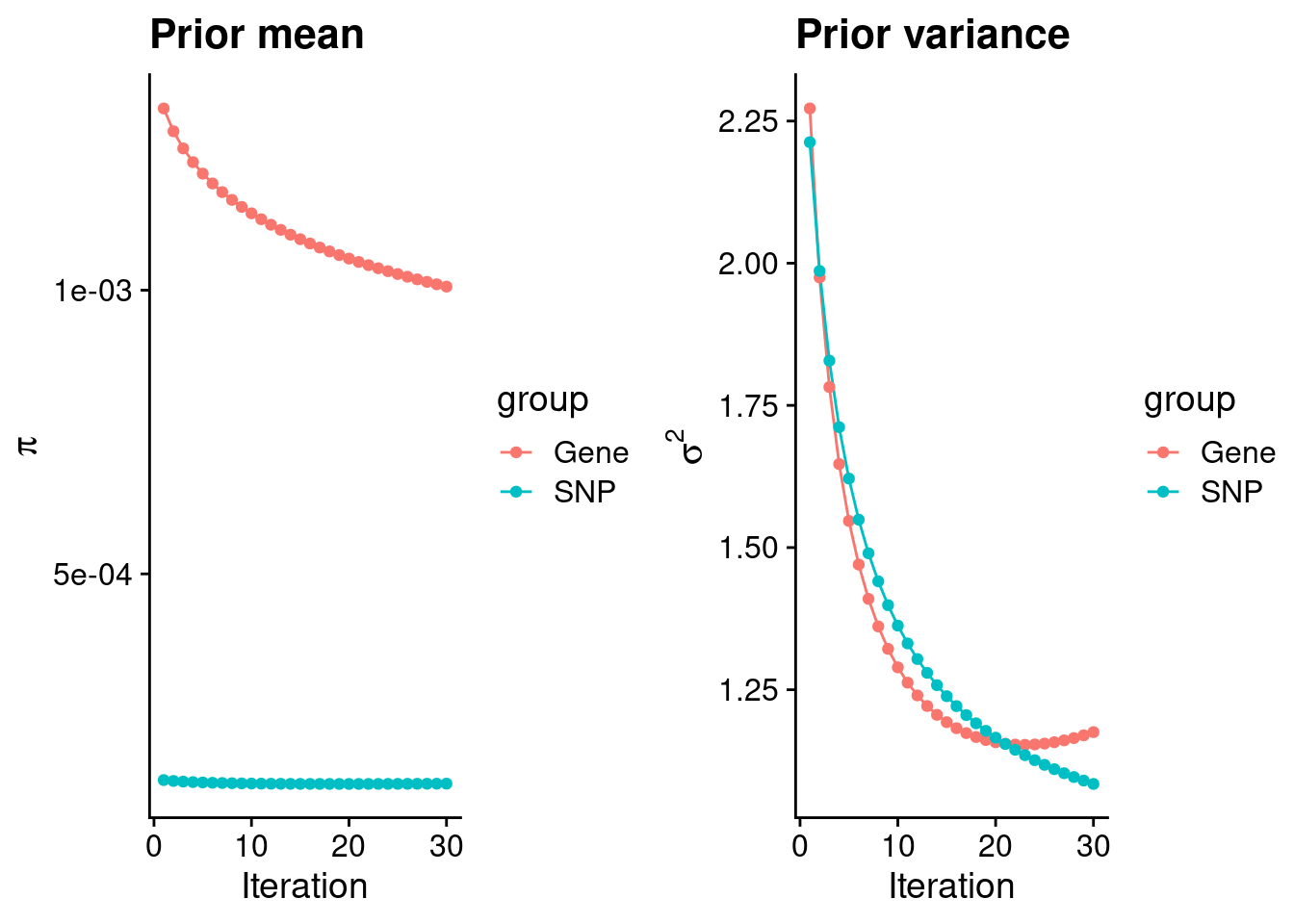
| Version | Author | Date |
|---|---|---|
| dfd2b5f | wesleycrouse | 2021-09-07 |
#estimated group prior
estimated_group_prior <- group_prior_rec[,ncol(group_prior_rec)]
names(estimated_group_prior) <- c("gene", "snp")
estimated_group_prior["snp"] <- estimated_group_prior["snp"]*thin #adjust parameter to account for thin argument
print(estimated_group_prior) gene snp
0.0010062774 0.0001304274 #estimated group prior variance
estimated_group_prior_var <- group_prior_var_rec[,ncol(group_prior_var_rec)]
names(estimated_group_prior_var) <- c("gene", "snp")
print(estimated_group_prior_var) gene snp
1.175389 1.084307 #report sample size
print(sample_size)[1] 54498#report group size
group_size <- c(nrow(ctwas_gene_res), n_snps)
print(group_size)[1] 11095 8697330#estimated group PVE
estimated_group_pve <- estimated_group_prior_var*estimated_group_prior*group_size/sample_size #check PVE calculation
names(estimated_group_pve) <- c("gene", "snp")
print(estimated_group_pve) gene snp
0.0002407942 0.0225697307 #compare sum(PIP*mu2/sample_size) with above PVE calculation
c(sum(ctwas_gene_res$PVE),sum(ctwas_snp_res$PVE))[1] 0.004927462 0.466129415Genes with highest PIPs
#distribution of PIPs
hist(ctwas_gene_res$susie_pip, xlim=c(0,1), main="Distribution of Gene PIPs")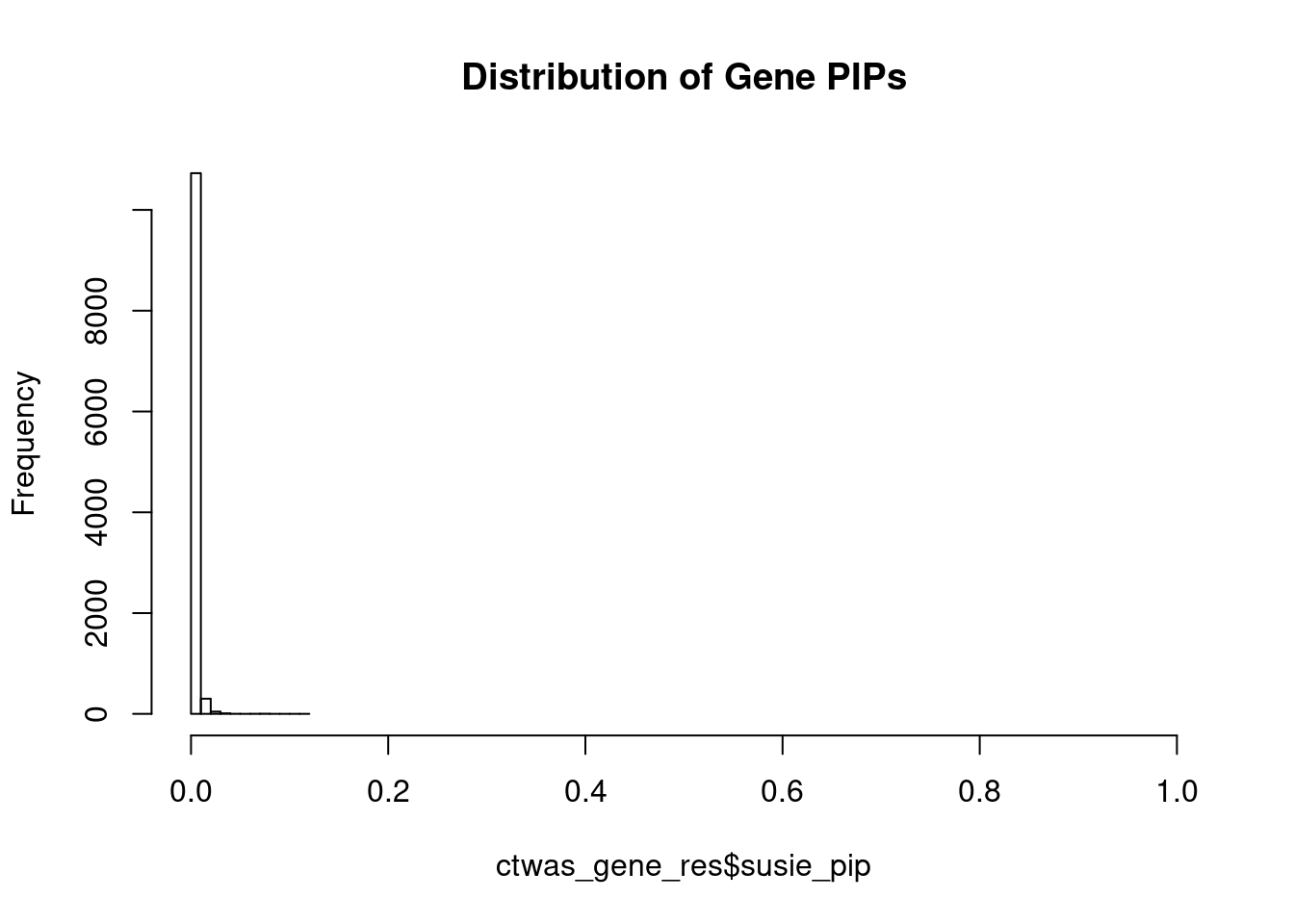
| Version | Author | Date |
|---|---|---|
| dfd2b5f | wesleycrouse | 2021-09-07 |
#genes with PIP>0.8 or 20 highest PIPs
head(ctwas_gene_res[order(-ctwas_gene_res$susie_pip),report_cols], max(sum(ctwas_gene_res$susie_pip>0.8), 20)) genename region_tag susie_pip mu2 PVE z
4871 EPRS 1_112 0.119 22.34 4.9e-05 4.08
4142 GAMT 19_2 0.077 19.85 2.8e-05 -3.81
6002 GTF3C5 9_70 0.075 19.01 2.6e-05 -3.46
3122 AGMAT 1_11 0.074 19.48 2.7e-05 -3.57
4093 ATP1B2 17_7 0.069 20.83 2.6e-05 -4.72
2587 LIN7A 12_49 0.066 18.66 2.3e-05 3.42
4992 NUMA1 11_40 0.053 17.61 1.7e-05 3.42
82 GDE1 16_18 0.047 17.14 1.5e-05 -3.69
7164 SLC16A14 2_135 0.041 17.01 1.3e-05 -3.47
7143 TRIM17 1_116 0.039 15.68 1.1e-05 -3.23
6195 CCDC3 10_12 0.036 17.21 1.1e-05 3.20
4340 DNAJB1 19_12 0.036 15.32 1.0e-05 -3.11
5230 NUP58 13_6 0.034 15.36 9.7e-06 -3.00
1685 TCFL5 20_37 0.034 14.91 9.2e-06 -3.04
3979 VIL1 2_129 0.033 15.09 9.1e-06 -3.24
10836 HLA-C 6_26 0.033 25.29 1.5e-05 -4.99
4181 C6orf203 6_71 0.033 14.81 8.9e-06 3.09
2841 PDGFRB 5_88 0.032 14.55 8.4e-06 2.93
1424 MTAP 9_16 0.032 14.86 8.7e-06 2.95
11649 MIATNB 22_8 0.032 14.79 8.6e-06 2.93Genes with largest effect sizes
#plot PIP vs effect size
plot(ctwas_gene_res$susie_pip, ctwas_gene_res$mu2, xlab="PIP", ylab="mu^2", main="Gene PIPs vs Effect Size")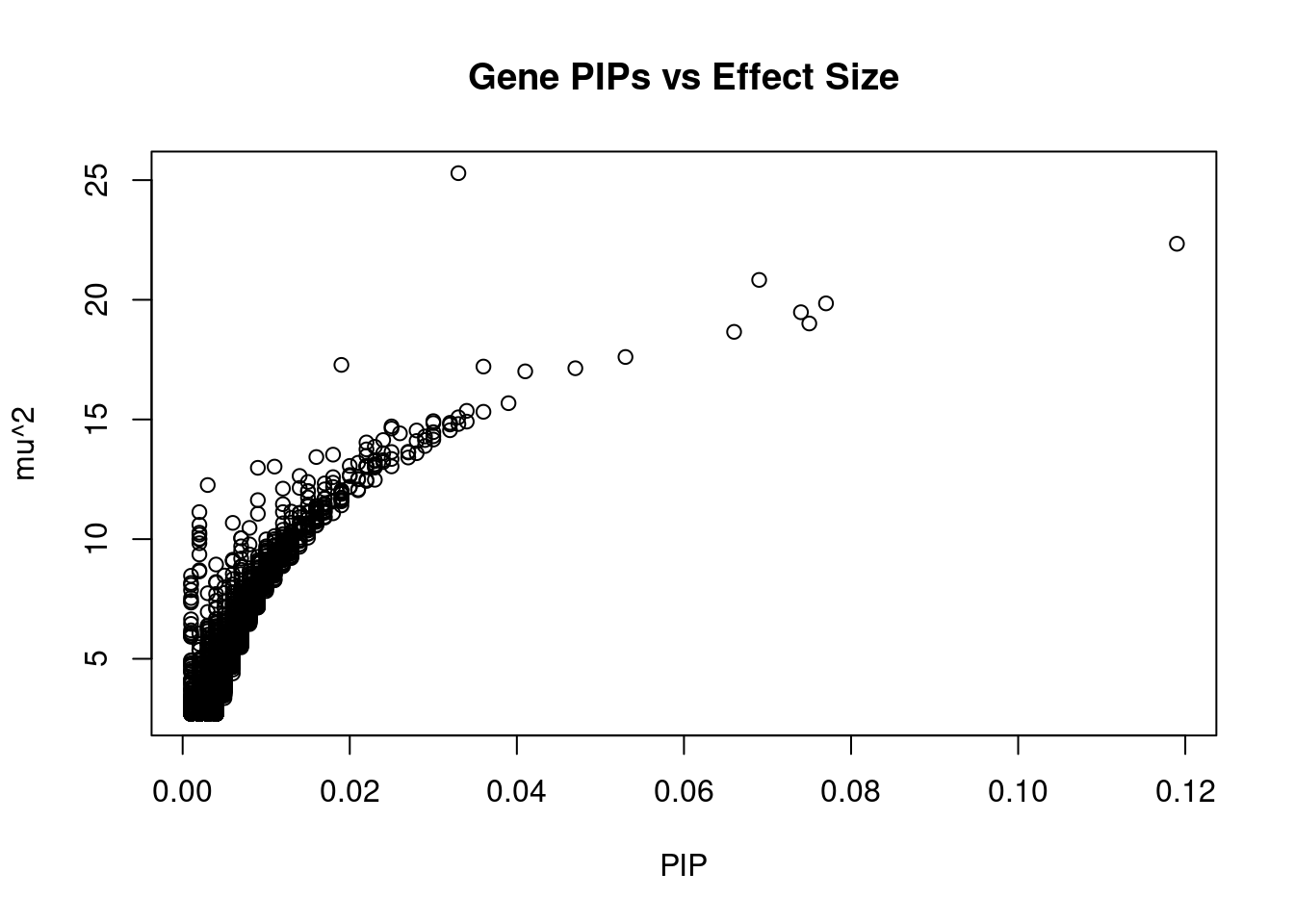
| Version | Author | Date |
|---|---|---|
| dfd2b5f | wesleycrouse | 2021-09-07 |
#genes with 20 largest effect sizes
head(ctwas_gene_res[order(-ctwas_gene_res$mu2),report_cols],20) genename region_tag susie_pip mu2 PVE z
10836 HLA-C 6_26 0.033 25.29 1.5e-05 -4.99
4871 EPRS 1_112 0.119 22.34 4.9e-05 4.08
4093 ATP1B2 17_7 0.069 20.83 2.6e-05 -4.72
4142 GAMT 19_2 0.077 19.85 2.8e-05 -3.81
3122 AGMAT 1_11 0.074 19.48 2.7e-05 -3.57
6002 GTF3C5 9_70 0.075 19.01 2.6e-05 -3.46
2587 LIN7A 12_49 0.066 18.66 2.3e-05 3.42
4992 NUMA1 11_40 0.053 17.61 1.7e-05 3.42
6527 ADAMTS3 4_52 0.019 17.28 6.0e-06 -3.25
6195 CCDC3 10_12 0.036 17.21 1.1e-05 3.20
82 GDE1 16_18 0.047 17.14 1.5e-05 -3.69
7164 SLC16A14 2_135 0.041 17.01 1.3e-05 -3.47
7143 TRIM17 1_116 0.039 15.68 1.1e-05 -3.23
5230 NUP58 13_6 0.034 15.36 9.7e-06 -3.00
4340 DNAJB1 19_12 0.036 15.32 1.0e-05 -3.11
3979 VIL1 2_129 0.033 15.09 9.1e-06 -3.24
7389 MAD2L1 4_78 0.030 14.93 8.1e-06 2.96
1685 TCFL5 20_37 0.034 14.91 9.2e-06 -3.04
1424 MTAP 9_16 0.032 14.86 8.7e-06 2.95
6970 PGAP3 17_23 0.030 14.85 8.2e-06 -3.42Genes with highest PVE
#genes with 20 highest pve
head(ctwas_gene_res[order(-ctwas_gene_res$PVE),report_cols],20) genename region_tag susie_pip mu2 PVE z
4871 EPRS 1_112 0.119 22.34 4.9e-05 4.08
4142 GAMT 19_2 0.077 19.85 2.8e-05 -3.81
3122 AGMAT 1_11 0.074 19.48 2.7e-05 -3.57
6002 GTF3C5 9_70 0.075 19.01 2.6e-05 -3.46
4093 ATP1B2 17_7 0.069 20.83 2.6e-05 -4.72
2587 LIN7A 12_49 0.066 18.66 2.3e-05 3.42
4992 NUMA1 11_40 0.053 17.61 1.7e-05 3.42
10836 HLA-C 6_26 0.033 25.29 1.5e-05 -4.99
82 GDE1 16_18 0.047 17.14 1.5e-05 -3.69
7164 SLC16A14 2_135 0.041 17.01 1.3e-05 -3.47
7143 TRIM17 1_116 0.039 15.68 1.1e-05 -3.23
6195 CCDC3 10_12 0.036 17.21 1.1e-05 3.20
4340 DNAJB1 19_12 0.036 15.32 1.0e-05 -3.11
5230 NUP58 13_6 0.034 15.36 9.7e-06 -3.00
1685 TCFL5 20_37 0.034 14.91 9.2e-06 -3.04
3979 VIL1 2_129 0.033 15.09 9.1e-06 -3.24
4181 C6orf203 6_71 0.033 14.81 8.9e-06 3.09
1424 MTAP 9_16 0.032 14.86 8.7e-06 2.95
11649 MIATNB 22_8 0.032 14.79 8.6e-06 2.93
2841 PDGFRB 5_88 0.032 14.55 8.4e-06 2.93Genes with largest z scores
#genes with 20 largest z scores
head(ctwas_gene_res[order(-abs(ctwas_gene_res$z)),report_cols],20) genename region_tag susie_pip mu2 PVE z
10836 HLA-C 6_26 0.033 25.29 1.5e-05 -4.99
4093 ATP1B2 17_7 0.069 20.83 2.6e-05 -4.72
4871 EPRS 1_112 0.119 22.34 4.9e-05 4.08
7001 ALOX15 17_4 0.024 14.14 6.1e-06 4.00
5423 ARRB2 17_4 0.022 13.75 5.6e-06 -3.96
4142 GAMT 19_2 0.077 19.85 2.8e-05 -3.81
11526 TNFSF12 17_7 0.012 11.46 2.6e-06 3.75
82 GDE1 16_18 0.047 17.14 1.5e-05 -3.69
3122 AGMAT 1_11 0.074 19.48 2.7e-05 -3.57
7164 SLC16A14 2_135 0.041 17.01 1.3e-05 -3.47
6002 GTF3C5 9_70 0.075 19.01 2.6e-05 -3.46
4992 NUMA1 11_40 0.053 17.61 1.7e-05 3.42
2587 LIN7A 12_49 0.066 18.66 2.3e-05 3.42
6970 PGAP3 17_23 0.030 14.85 8.2e-06 -3.42
5463 MIEN1 17_23 0.028 14.54 7.6e-06 3.38
6527 ADAMTS3 4_52 0.019 17.28 6.0e-06 -3.25
3979 VIL1 2_129 0.033 15.09 9.1e-06 -3.24
7143 TRIM17 1_116 0.039 15.68 1.1e-05 -3.23
11997 LINC01963 2_127 0.030 14.47 7.8e-06 -3.21
4269 ATP6V1E1 22_2 0.020 13.06 4.9e-06 -3.21Comparing z scores and PIPs
#set nominal signifiance threshold for z scores
alpha <- 0.05
#bonferroni adjusted threshold for z scores
sig_thresh <- qnorm(1-(alpha/nrow(ctwas_gene_res)/2), lower=T)
#Q-Q plot for z scores
obs_z <- ctwas_gene_res$z[order(ctwas_gene_res$z)]
exp_z <- qnorm((1:nrow(ctwas_gene_res))/nrow(ctwas_gene_res))
plot(exp_z, obs_z, xlab="Expected z", ylab="Observed z", main="Gene z score Q-Q plot")
abline(a=0,b=1)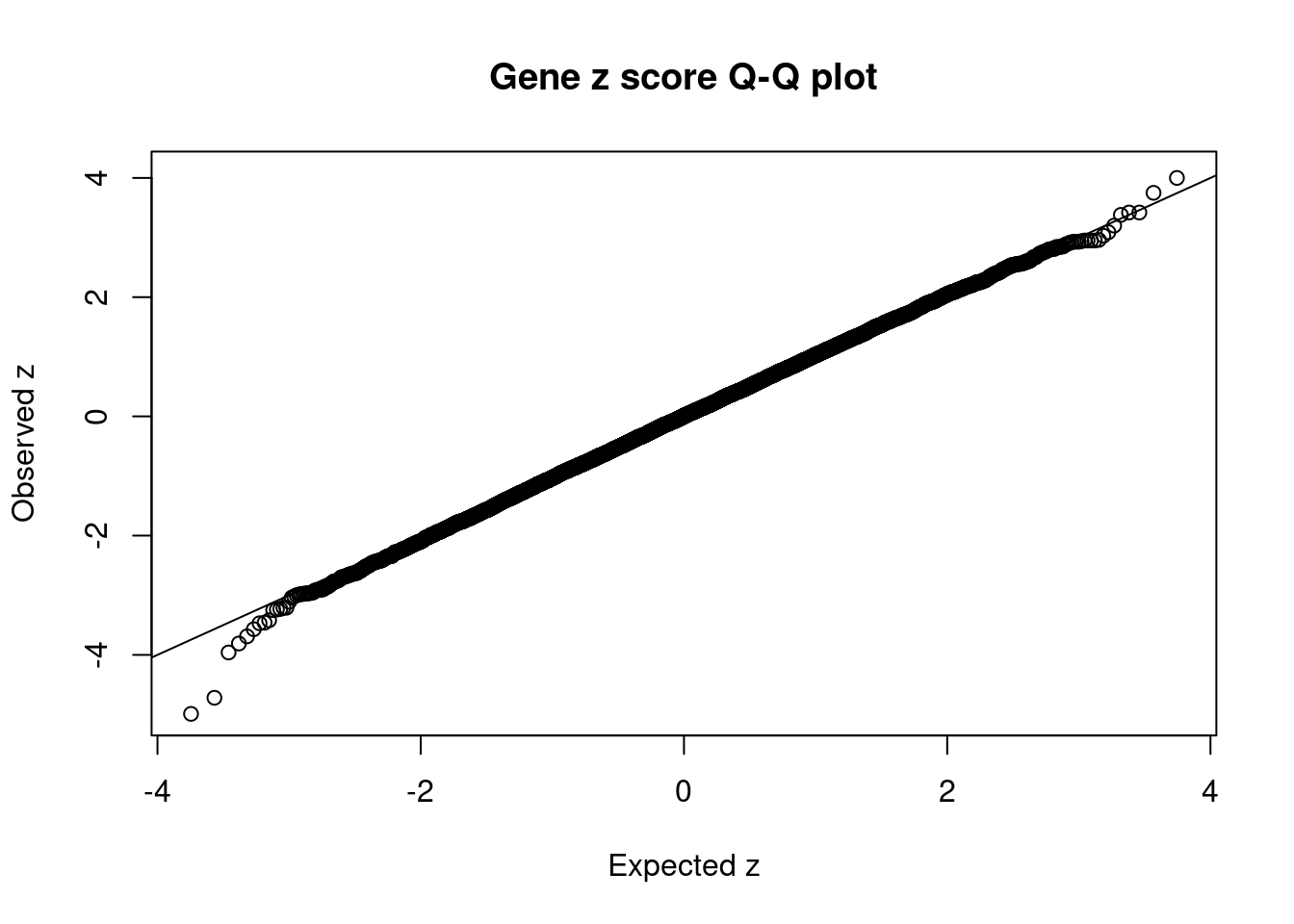
| Version | Author | Date |
|---|---|---|
| dfd2b5f | wesleycrouse | 2021-09-07 |
#plot z score vs PIP
plot(abs(ctwas_gene_res$z), ctwas_gene_res$susie_pip, xlab="abs(z)", ylab="PIP")
abline(v=sig_thresh, col="red", lty=2)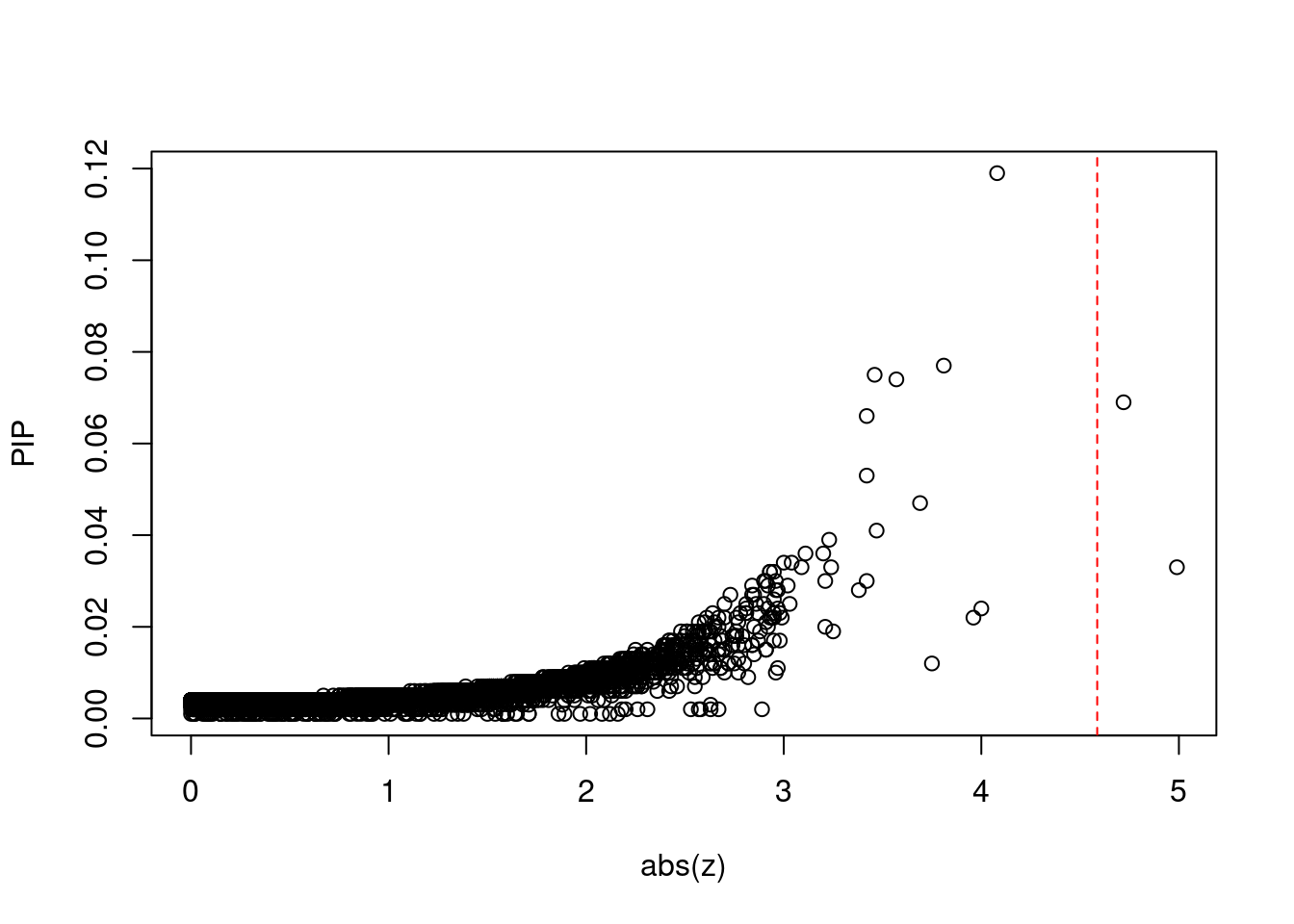
| Version | Author | Date |
|---|---|---|
| dfd2b5f | wesleycrouse | 2021-09-07 |
#proportion of significant z scores
mean(abs(ctwas_gene_res$z) > sig_thresh)[1] 0.0001802614#genes with most significant z scores
head(ctwas_gene_res[order(-abs(ctwas_gene_res$z)),report_cols],20) genename region_tag susie_pip mu2 PVE z
10836 HLA-C 6_26 0.033 25.29 1.5e-05 -4.99
4093 ATP1B2 17_7 0.069 20.83 2.6e-05 -4.72
4871 EPRS 1_112 0.119 22.34 4.9e-05 4.08
7001 ALOX15 17_4 0.024 14.14 6.1e-06 4.00
5423 ARRB2 17_4 0.022 13.75 5.6e-06 -3.96
4142 GAMT 19_2 0.077 19.85 2.8e-05 -3.81
11526 TNFSF12 17_7 0.012 11.46 2.6e-06 3.75
82 GDE1 16_18 0.047 17.14 1.5e-05 -3.69
3122 AGMAT 1_11 0.074 19.48 2.7e-05 -3.57
7164 SLC16A14 2_135 0.041 17.01 1.3e-05 -3.47
6002 GTF3C5 9_70 0.075 19.01 2.6e-05 -3.46
4992 NUMA1 11_40 0.053 17.61 1.7e-05 3.42
2587 LIN7A 12_49 0.066 18.66 2.3e-05 3.42
6970 PGAP3 17_23 0.030 14.85 8.2e-06 -3.42
5463 MIEN1 17_23 0.028 14.54 7.6e-06 3.38
6527 ADAMTS3 4_52 0.019 17.28 6.0e-06 -3.25
3979 VIL1 2_129 0.033 15.09 9.1e-06 -3.24
7143 TRIM17 1_116 0.039 15.68 1.1e-05 -3.23
11997 LINC01963 2_127 0.030 14.47 7.8e-06 -3.21
4269 ATP6V1E1 22_2 0.020 13.06 4.9e-06 -3.21Locus plots for genes and SNPs
ctwas_gene_res_sortz <- ctwas_gene_res[order(-abs(ctwas_gene_res$z)),]
n_plots <- 5
for (region_tag_plot in head(unique(ctwas_gene_res_sortz$region_tag), n_plots)){
ctwas_res_region <- ctwas_res[ctwas_res$region_tag==region_tag_plot,]
start <- min(ctwas_res_region$pos)
end <- max(ctwas_res_region$pos)
ctwas_res_region <- ctwas_res_region[order(ctwas_res_region$pos),]
ctwas_res_region_gene <- ctwas_res_region[ctwas_res_region$type=="gene",]
ctwas_res_region_snp <- ctwas_res_region[ctwas_res_region$type=="SNP",]
#region name
print(paste0("Region: ", region_tag_plot))
#table of genes in region
print(ctwas_res_region_gene[,report_cols])
par(mfrow=c(4,1))
#gene z scores
plot(ctwas_res_region_gene$pos, abs(ctwas_res_region_gene$z), xlab="Position", ylab="abs(gene_z)", xlim=c(start,end),
ylim=c(0,max(sig_thresh, abs(ctwas_res_region_gene$z))),
main=paste0("Region: ", region_tag_plot))
abline(h=sig_thresh,col="red",lty=2)
#significance threshold for SNPs
alpha_snp <- 5*10^(-8)
sig_thresh_snp <- qnorm(1-alpha_snp/2, lower=T)
#snp z scores
plot(ctwas_res_region_snp$pos, abs(ctwas_res_region_snp$z), xlab="Position", ylab="abs(snp_z)",xlim=c(start,end),
ylim=c(0,max(sig_thresh_snp, max(abs(ctwas_res_region_snp$z)))))
abline(h=sig_thresh_snp,col="purple",lty=2)
#gene pips
plot(ctwas_res_region_gene$pos, ctwas_res_region_gene$susie_pip, xlab="Position", ylab="Gene PIP", xlim=c(start,end), ylim=c(0,1))
abline(h=0.8,col="blue",lty=2)
#snp pips
plot(ctwas_res_region_snp$pos, ctwas_res_region_snp$susie_pip, xlab="Position", ylab="SNP PIP", xlim=c(start,end), ylim=c(0,1))
abline(h=0.8,col="blue",lty=2)
}[1] "Region: 6_26"
genename region_tag susie_pip mu2 PVE z
10855 HLA-G 6_26 0.001 3.19 3.3e-08 0.80
12599 HCP5B 6_26 0.002 10.60 4.3e-07 -2.67
10968 HLA-A 6_26 0.001 4.80 6.7e-08 1.35
10853 HCG9 6_26 0.001 4.91 7.0e-08 1.38
10851 PPP1R11 6_26 0.001 3.32 3.5e-08 0.81
661 ZNRD1 6_26 0.001 2.72 2.6e-08 0.15
10850 RNF39 6_26 0.001 3.69 4.2e-08 -0.86
10848 TRIM10 6_26 0.002 10.19 3.8e-07 2.58
10847 TRIM15 6_26 0.001 2.74 2.6e-08 -0.15
11418 TRIM26 6_26 0.001 3.54 3.9e-08 -0.89
10845 TRIM39 6_26 0.001 6.05 1.1e-07 1.71
11563 RPP21 6_26 0.001 3.40 3.7e-08 -0.68
10844 HLA-E 6_26 0.001 3.96 4.7e-08 -1.10
10841 MRPS18B 6_26 0.001 3.66 4.1e-08 0.90
10840 C6orf136 6_26 0.002 10.02 3.7e-07 2.53
10839 DHX16 6_26 0.001 3.95 4.7e-08 -0.91
5868 PPP1R18 6_26 0.001 3.04 3.1e-08 -0.63
4976 NRM 6_26 0.001 2.85 2.8e-08 -0.28
4970 FLOT1 6_26 0.001 6.46 1.2e-07 -1.65
10230 TUBB 6_26 0.001 2.86 2.8e-08 0.32
4971 IER3 6_26 0.001 3.19 3.3e-08 -0.59
11120 LINC00243 6_26 0.001 4.69 6.4e-08 -1.32
10843 DDR1 6_26 0.001 3.26 3.4e-08 0.59
11052 GTF2H4 6_26 0.001 3.20 3.3e-08 0.73
4978 VARS2 6_26 0.001 4.94 7.1e-08 1.58
10838 CCHCR1 6_26 0.002 9.83 3.5e-07 -2.63
4969 TCF19 6_26 0.001 2.70 2.6e-08 -0.08
10966 HCG27 6_26 0.002 10.27 3.9e-07 2.89
10837 POU5F1 6_26 0.001 3.00 3.0e-08 -0.47
10836 HLA-C 6_26 0.033 25.29 1.5e-05 -4.99
10788 NOTCH4 6_26 0.001 2.79 2.7e-08 0.21
11439 HLA-B 6_26 0.001 2.81 2.7e-08 -0.42
12270 XXbac-BPG181B23.7 6_26 0.001 2.90 2.8e-08 -0.47
10834 MICA 6_26 0.001 3.17 3.3e-08 -0.63
10833 MICB 6_26 0.001 3.25 3.4e-08 -0.43
10830 LST1 6_26 0.001 2.70 2.6e-08 0.00
10619 DDX39B 6_26 0.001 3.15 3.2e-08 0.63
11050 ATP6V1G2 6_26 0.001 4.49 5.9e-08 1.50
10831 NFKBIL1 6_26 0.001 2.72 2.6e-08 0.11
11282 LTA 6_26 0.001 2.98 3.0e-08 0.52
11296 LTB 6_26 0.001 2.99 3.0e-08 0.53
11395 TNF 6_26 0.001 4.43 5.8e-08 -1.09
10829 NCR3 6_26 0.001 4.15 5.1e-08 -1.14
10828 AIF1 6_26 0.001 4.73 6.5e-08 1.16
10827 PRRC2A 6_26 0.001 5.94 1.0e-07 1.60
10826 BAG6 6_26 0.001 2.87 2.8e-08 -0.35
10825 APOM 6_26 0.002 11.13 5.0e-07 2.57
10824 C6orf47 6_26 0.001 4.49 5.9e-08 1.16
10822 CSNK2B 6_26 0.001 6.65 1.3e-07 -1.71
10823 GPANK1 6_26 0.001 5.92 1.0e-07 1.58
11539 LY6G5B 6_26 0.001 2.76 2.6e-08 -0.27
10821 LY6G5C 6_26 0.001 2.72 2.6e-08 0.07
11639 LY6G6D 6_26 0.001 2.91 2.9e-08 -0.44
10818 MPIG6B 6_26 0.001 2.82 2.7e-08 0.30
10819 LY6G6C 6_26 0.001 4.81 6.7e-08 -1.26
11048 DDAH2 6_26 0.001 4.58 6.1e-08 -1.23
10817 MSH5 6_26 0.001 4.03 4.9e-08 -0.98
11047 CLIC1 6_26 0.002 9.36 3.0e-07 2.31
11327 SAPCD1 6_26 0.003 12.26 6.8e-07 -2.63
10814 VWA7 6_26 0.001 3.46 3.8e-08 -0.73
10809 C6orf48 6_26 0.001 4.11 5.0e-08 -1.01
10813 VARS 6_26 0.001 2.79 2.7e-08 -0.24
10812 LSM2 6_26 0.001 2.84 2.8e-08 -0.32
10811 HSPA1L 6_26 0.001 2.71 2.6e-08 -0.06
10808 NEU1 6_26 0.002 8.70 2.5e-07 2.20
10807 SLC44A4 6_26 0.002 8.65 2.5e-07 -2.18
7712 C2 6_26 0.001 8.47 2.3e-07 -2.16
10805 EHMT2 6_26 0.001 7.35 1.6e-07 -1.89
10802 NELFE 6_26 0.001 5.98 1.0e-07 -1.54
10801 SKIV2L 6_26 0.001 6.19 1.1e-07 1.64
10797 STK19 6_26 0.002 9.99 3.6e-07 2.26
10800 DXO 6_26 0.001 3.74 4.3e-08 0.84
11652 C4A 6_26 0.001 8.17 2.1e-07 2.12
11218 C4B 6_26 0.001 7.42 1.7e-07 -1.97
11374 CYP21A2 6_26 0.001 2.78 2.7e-08 0.15
11193 PPT2 6_26 0.001 4.11 5.0e-08 -1.08
11043 ATF6B 6_26 0.001 7.54 1.7e-07 1.86
10795 FKBPL 6_26 0.001 2.99 3.0e-08 -0.41
10794 PRRT1 6_26 0.001 3.03 3.0e-08 0.46
10791 RNF5 6_26 0.001 2.89 2.8e-08 0.32
11565 EGFL8 6_26 0.001 6.09 1.1e-07 -1.58
10792 AGPAT1 6_26 0.001 3.44 3.7e-08 -0.70
10790 AGER 6_26 0.001 8.09 2.1e-07 -2.08
10789 PBX2 6_26 0.001 2.78 2.7e-08 0.32
10608 HLA-DRB5 6_26 0.001 7.87 1.9e-07 -2.02
10325 HLA-DQA1 6_26 0.001 3.81 4.4e-08 -0.92
11490 HLA-DQA2 6_26 0.001 3.25 3.4e-08 0.72
11389 HLA-DQB2 6_26 0.001 2.86 2.8e-08 -0.33
9260 HLA-DQB1 6_26 0.001 2.77 2.7e-08 0.22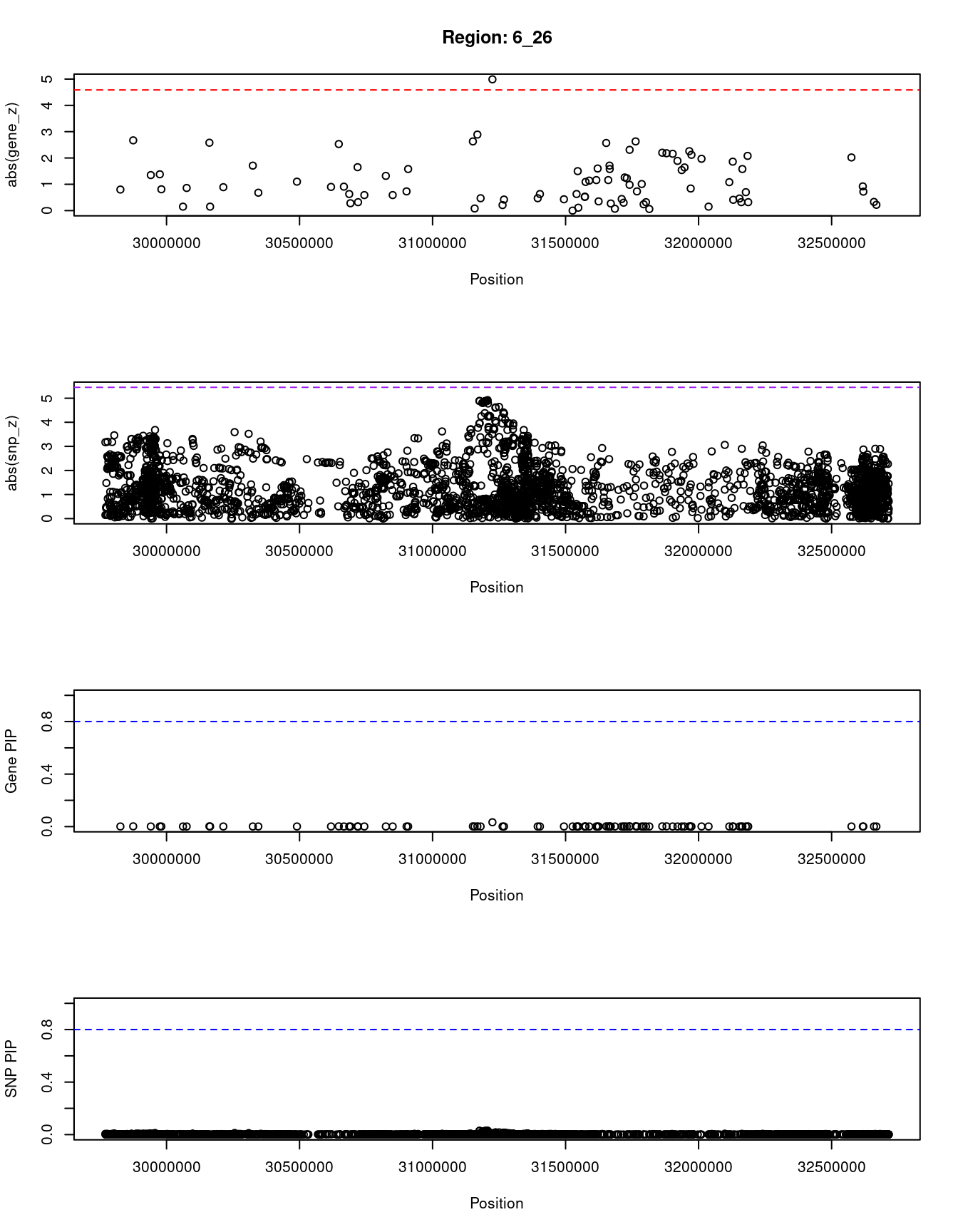
| Version | Author | Date |
|---|---|---|
| dfd2b5f | wesleycrouse | 2021-09-07 |
[1] "Region: 17_7"
genename region_tag susie_pip mu2 PVE z
7010 FGF11 17_7 0.014 12.14 3.1e-06 -2.59
8293 CHRNB1 17_7 0.002 2.75 1.3e-07 -0.39
9403 POLR2A 17_7 0.003 3.94 2.2e-07 1.40
11526 TNFSF12 17_7 0.012 11.46 2.6e-06 3.75
7008 TNFSF13 17_7 0.003 3.75 2.1e-07 -0.81
7009 SENP3 17_7 0.003 3.69 2.0e-07 -0.61
4096 MPDU1 17_7 0.003 4.18 2.5e-07 0.75
5427 SAT2 17_7 0.003 2.89 1.4e-07 0.35
4093 ATP1B2 17_7 0.069 20.83 2.6e-05 -4.72
5425 WRAP53 17_7 0.006 7.21 7.5e-07 -2.14
5430 TP53 17_7 0.003 3.43 1.8e-07 -0.53
4402 KDM6B 17_7 0.002 2.72 1.2e-07 -0.31
7989 TMEM88 17_7 0.003 4.11 2.4e-07 1.09
9555 NAA38 17_7 0.005 5.97 5.0e-07 1.57
8272 CHD3 17_7 0.003 2.89 1.4e-07 -0.39
9286 AC025335.1 17_7 0.003 3.59 1.9e-07 0.90
8279 KCNAB3 17_7 0.002 2.70 1.2e-07 -0.07
8277 CNTROB 17_7 0.003 2.93 1.4e-07 -0.44
8278 TRAPPC1 17_7 0.003 2.91 1.4e-07 -0.34
11172 VAMP2 17_7 0.002 2.71 1.2e-07 0.05
9234 TMEM107 17_7 0.003 4.22 2.5e-07 1.01
10292 BORCS6 17_7 0.002 2.75 1.3e-07 0.17
9228 LINC00324 17_7 0.002 2.70 1.2e-07 0.04
9218 PFAS 17_7 0.015 12.39 3.4e-06 2.60
9226 CTC1 17_7 0.002 2.74 1.3e-07 0.19
3790 SLC25A35 17_7 0.003 2.78 1.3e-07 0.24
9716 KRBA2 17_7 0.003 2.83 1.3e-07 -0.33
7011 RPL26 17_7 0.003 4.27 2.6e-07 1.07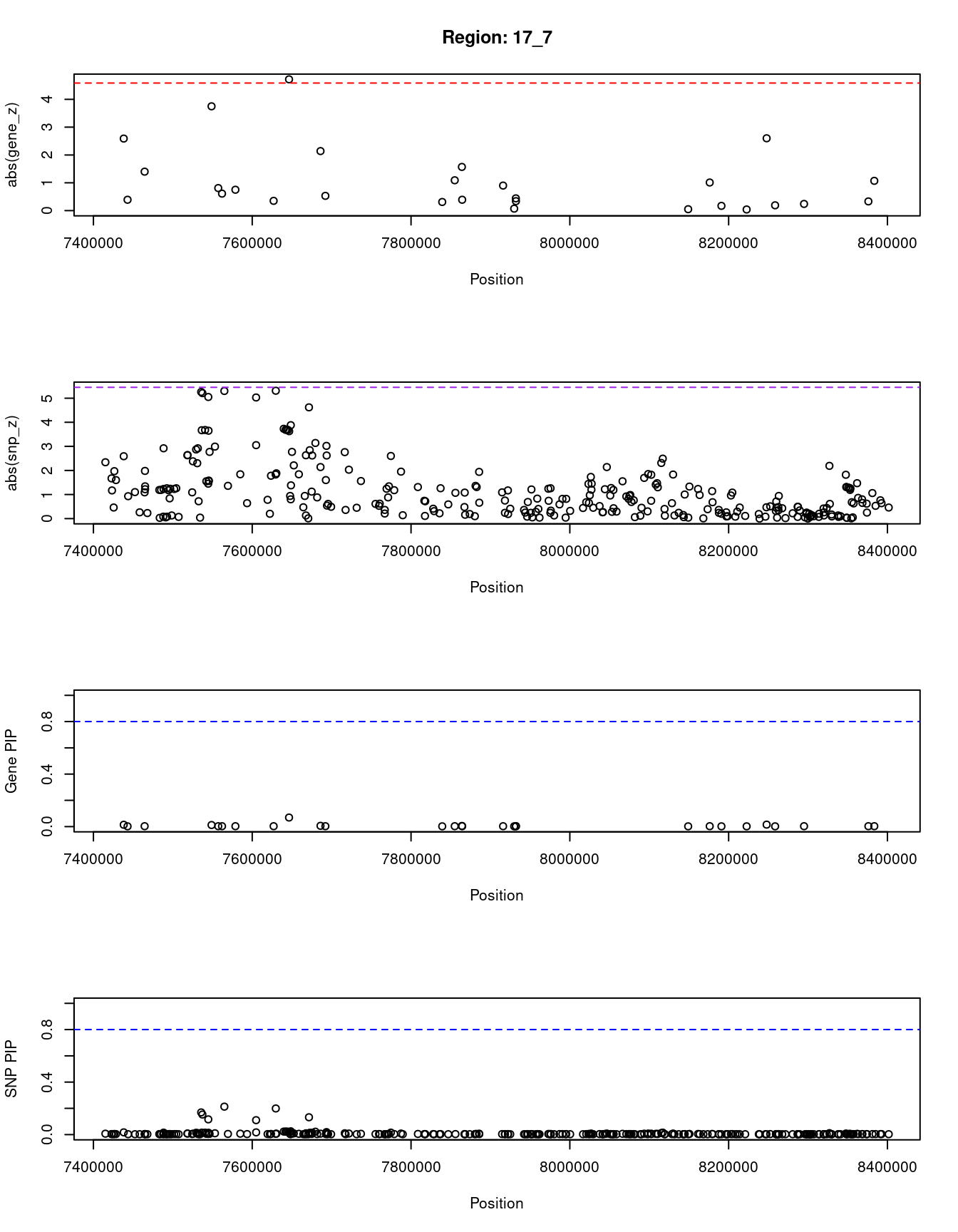
| Version | Author | Date |
|---|---|---|
| dfd2b5f | wesleycrouse | 2021-09-07 |
[1] "Region: 1_112"
genename region_tag susie_pip mu2 PVE z
4871 EPRS 1_112 0.119 22.34 4.9e-05 4.08
7125 BPNT1 1_112 0.003 2.78 1.7e-07 0.25
691 IARS2 1_112 0.003 2.93 1.9e-07 0.36
3289 RAB3GAP2 1_112 0.003 2.96 1.9e-07 0.50
7126 C1orf115 1_112 0.007 6.45 7.8e-07 1.66
3225 MARC2 1_112 0.004 3.47 2.4e-07 -0.69
9905 MARC1 1_112 0.003 2.99 1.9e-07 -0.44
11173 RNU6ATAC35P 1_112 0.004 3.80 2.8e-07 0.93
11507 LINC01352 1_112 0.005 4.77 4.3e-07 1.24
12333 RP11-295M18.6 1_112 0.004 3.71 2.7e-07 0.87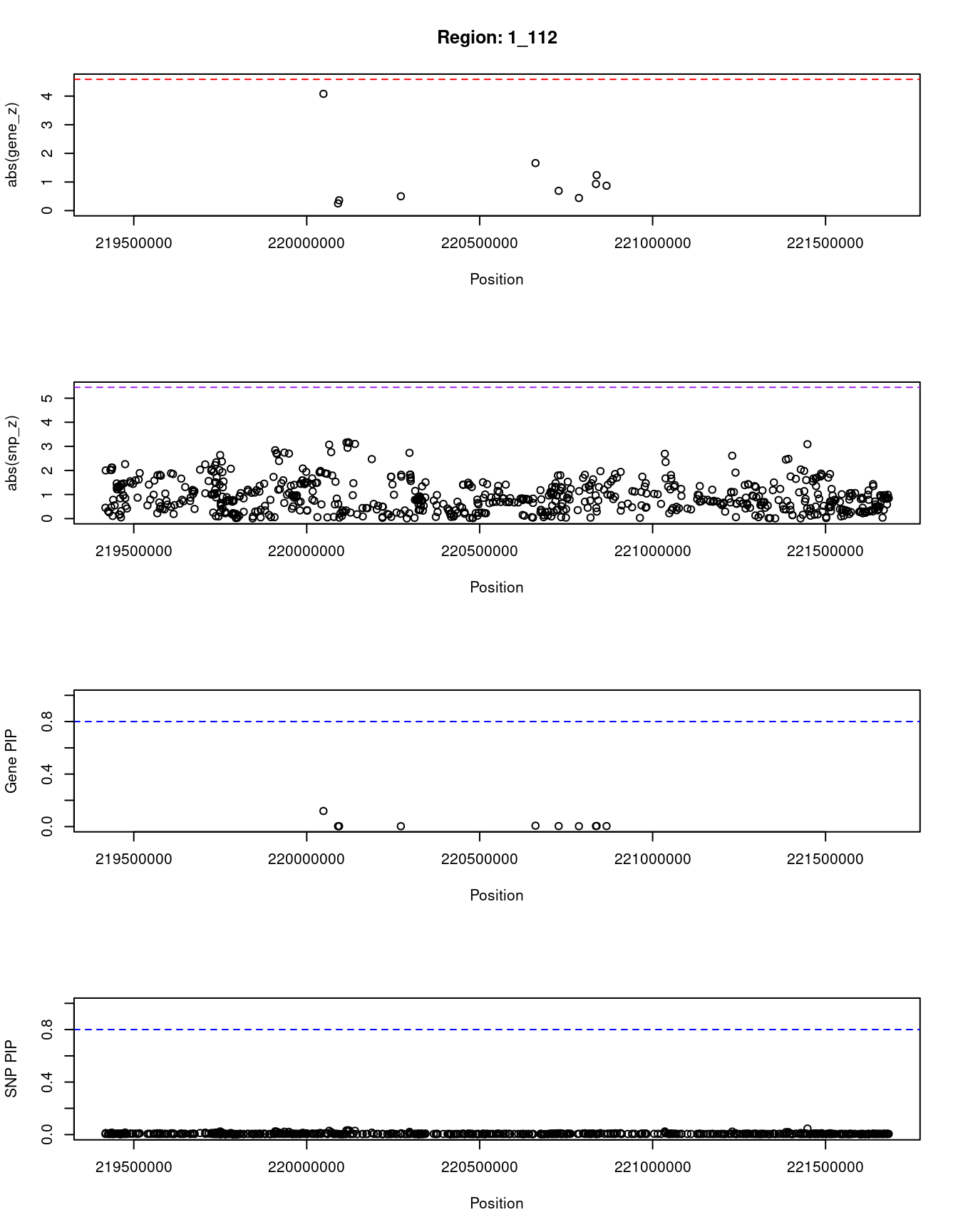
| Version | Author | Date |
|---|---|---|
| dfd2b5f | wesleycrouse | 2021-09-07 |
[1] "Region: 17_4"
genename region_tag susie_pip mu2 PVE z
1058 ITGAE 17_4 0.005 5.68 5.2e-07 1.42
850 NCBP3 17_4 0.003 3.44 2.1e-07 0.72
39 CAMKK1 17_4 0.007 7.78 1.0e-06 -1.87
2367 P2RX1 17_4 0.003 2.95 1.6e-07 0.42
851 ATP2A3 17_4 0.003 3.15 1.8e-07 0.59
861 ZZEF1 17_4 0.003 2.81 1.5e-07 -0.33
7964 CYB5D2 17_4 0.003 3.76 2.4e-07 -0.96
4383 UBE2G1 17_4 0.005 5.35 4.6e-07 1.49
9512 SPNS3 17_4 0.005 5.32 4.6e-07 -1.32
9556 SPNS2 17_4 0.005 5.61 5.1e-07 1.47
4380 MYBBP1A 17_4 0.003 3.49 2.1e-07 0.69
7001 ALOX15 17_4 0.024 14.14 6.1e-06 4.00
5423 ARRB2 17_4 0.022 13.75 5.6e-06 -3.96
5424 ZMYND15 17_4 0.004 5.10 4.2e-07 -1.78
7004 CXCL16 17_4 0.003 3.65 2.3e-07 -1.13
7003 MED11 17_4 0.003 3.35 2.0e-07 -0.96
9494 GLTPD2 17_4 0.013 10.89 2.6e-06 2.46
9538 VMO1 17_4 0.006 6.37 6.6e-07 -1.71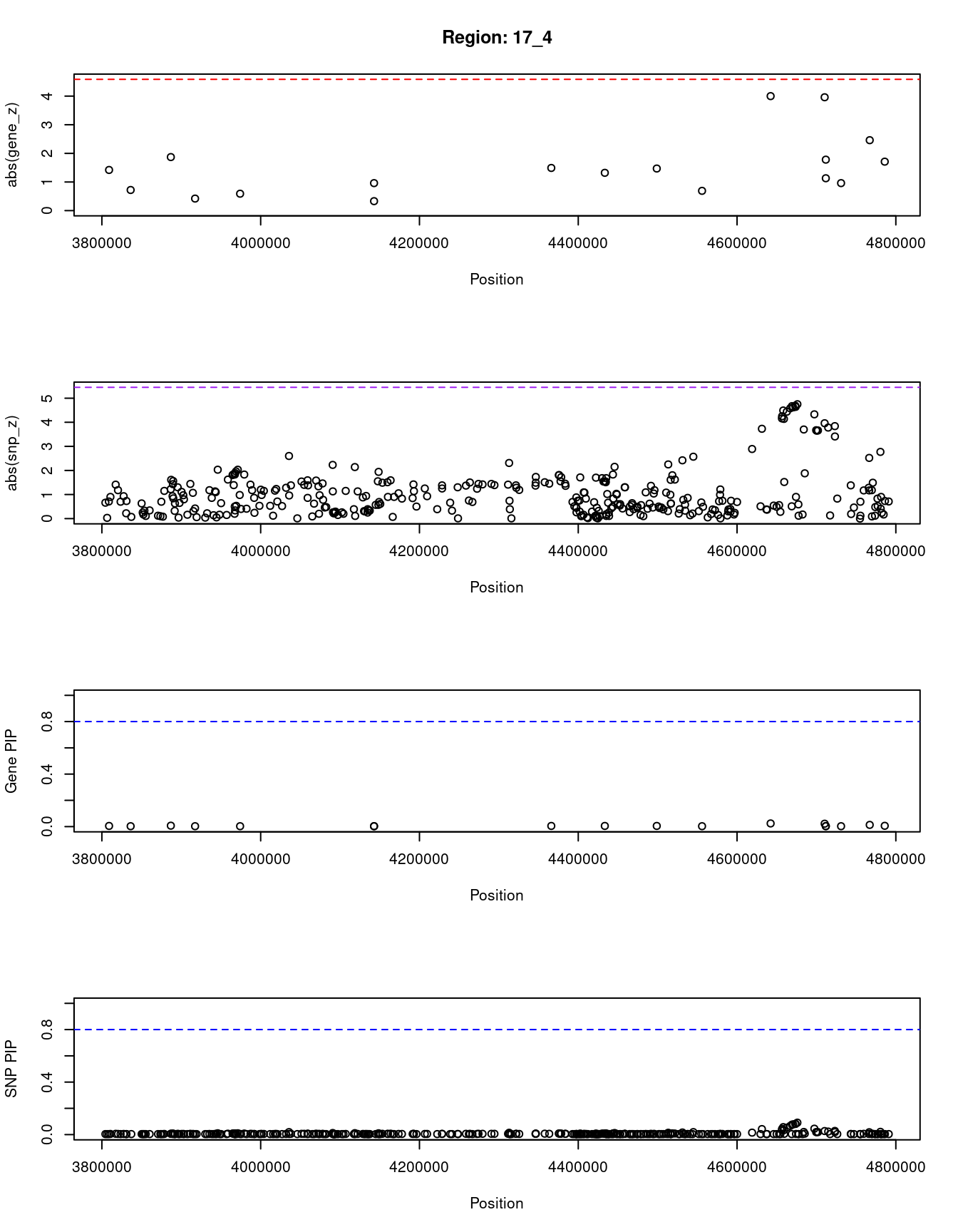
| Version | Author | Date |
|---|---|---|
| dfd2b5f | wesleycrouse | 2021-09-07 |
[1] "Region: 19_2"
genename region_tag susie_pip mu2 PVE z
1427 POLRMT 19_2 0.004 3.78 2.8e-07 0.88
12142 AC004156.3 19_2 0.004 3.18 2.1e-07 -0.58
750 FSTL3 19_2 0.004 4.15 3.3e-07 -1.00
9774 PRSS57 19_2 0.004 3.18 2.1e-07 0.58
1433 PALM 19_2 0.004 4.29 3.5e-07 1.06
201 PTBP1 19_2 0.013 10.26 2.5e-06 -2.34
10454 ELANE 19_2 0.005 4.49 3.8e-07 -1.13
10484 CFD 19_2 0.005 4.67 4.1e-07 -1.22
8877 MED16 19_2 0.004 3.75 2.8e-07 -0.91
3051 KISS1R 19_2 0.006 5.62 5.9e-07 -1.48
3053 ARID3A 19_2 0.014 10.36 2.6e-06 -2.36
9465 TMEM259 19_2 0.006 5.79 6.3e-07 1.52
602 CNN2 19_2 0.007 6.75 8.7e-07 1.73
603 ABCA7 19_2 0.005 4.36 3.6e-07 1.15
3055 GRIN3B 19_2 0.004 3.30 2.3e-07 0.70
9341 ARHGAP45 19_2 0.003 2.70 1.7e-07 0.03
1426 POLR2E 19_2 0.004 3.22 2.2e-07 0.65
7901 GPX4 19_2 0.003 2.71 1.7e-07 -0.08
609 SBNO2 19_2 0.008 7.10 9.8e-07 1.76
1417 ATP5D 19_2 0.003 2.87 1.8e-07 0.33
11311 C19orf24 19_2 0.003 2.78 1.7e-07 -0.24
6939 MUM1 19_2 0.003 2.87 1.8e-07 -0.32
4142 GAMT 19_2 0.077 19.85 2.8e-05 -3.81
3336 C19orf25 19_2 0.003 2.75 1.7e-07 -0.19
2960 PCSK4 19_2 0.003 2.72 1.7e-07 0.13
2959 REEP6 19_2 0.003 2.70 1.7e-07 -0.01
783 MBD3 19_2 0.004 3.52 2.5e-07 0.76
3964 UQCR11 19_2 0.003 2.79 1.7e-07 -0.26
781 TCF3 19_2 0.006 5.47 5.6e-07 -1.40
4168 ATP8B3 19_2 0.004 3.02 2.0e-07 -0.48
980 REXO1 19_2 0.003 2.88 1.8e-07 0.37
12032 CTB-31O20.2 19_2 0.003 2.91 1.9e-07 0.37
11294 SCAMP4 19_2 0.004 3.41 2.4e-07 -0.70
4484 CSNK1G2 19_2 0.007 6.81 8.9e-07 -1.70
4479 BTBD2 19_2 0.004 3.70 2.7e-07 0.85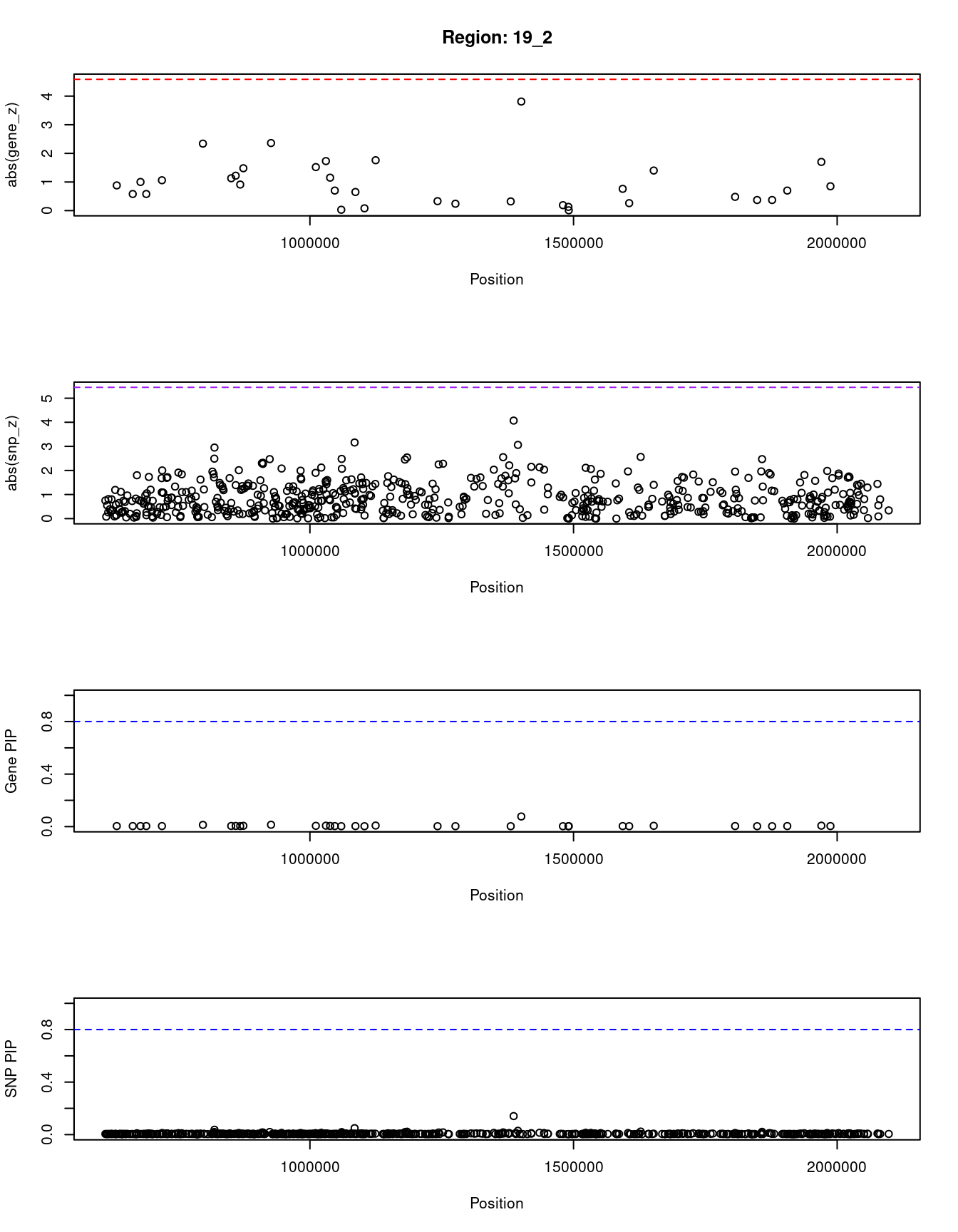
| Version | Author | Date |
|---|---|---|
| dfd2b5f | wesleycrouse | 2021-09-07 |
SNPs with highest PIPs
#snps with PIP>0.8 or 20 highest PIPs
head(ctwas_snp_res[order(-ctwas_snp_res$susie_pip),report_cols_snps],
max(sum(ctwas_snp_res$susie_pip>0.8), 20)) id region_tag susie_pip mu2 PVE z
396298 rs62444120 7_28 0.231 23.37 9.9e-05 -4.35
140037 rs75620930 3_1 0.230 23.43 9.9e-05 4.34
761470 rs4968212 17_7 0.213 24.80 9.7e-05 -5.30
476904 rs112371525 8_89 0.208 22.82 8.7e-05 4.23
761480 rs62059839 17_7 0.199 24.43 8.9e-05 5.31
679108 rs1959607 14_1 0.192 22.43 7.9e-05 4.21
526099 rs551737161 10_16 0.176 23.42 7.6e-05 3.60
813811 rs73000658 19_16 0.174 21.69 6.9e-05 -4.06
761458 rs9892862 17_7 0.169 23.48 7.3e-05 5.26
319306 rs13160700 5_104 0.166 21.87 6.7e-05 -4.06
165332 rs9813399 3_53 0.165 21.26 6.4e-05 4.14
70306 rs13429539 2_7 0.162 22.24 6.6e-05 -4.12
513886 rs10760461 9_65 0.158 21.52 6.2e-05 4.10
417774 rs876169 7_68 0.156 21.39 6.1e-05 4.31
761460 rs8073177 17_7 0.153 22.95 6.4e-05 5.21
272419 rs112157462 5_14 0.151 20.93 5.8e-05 3.98
459963 rs553564201 8_57 0.150 21.44 5.9e-05 3.99
154020 rs76750610 3_28 0.146 20.73 5.5e-05 -3.93
381048 rs113784847 7_3 0.143 21.31 5.6e-05 -4.04
2845 rs2274332 1_6 0.141 20.57 5.3e-05 -3.89SNPs with largest effect sizes
#plot PIP vs effect size
plot(ctwas_snp_res$susie_pip, ctwas_snp_res$mu2, xlab="PIP", ylab="mu^2", main="SNP PIPs vs Effect Size")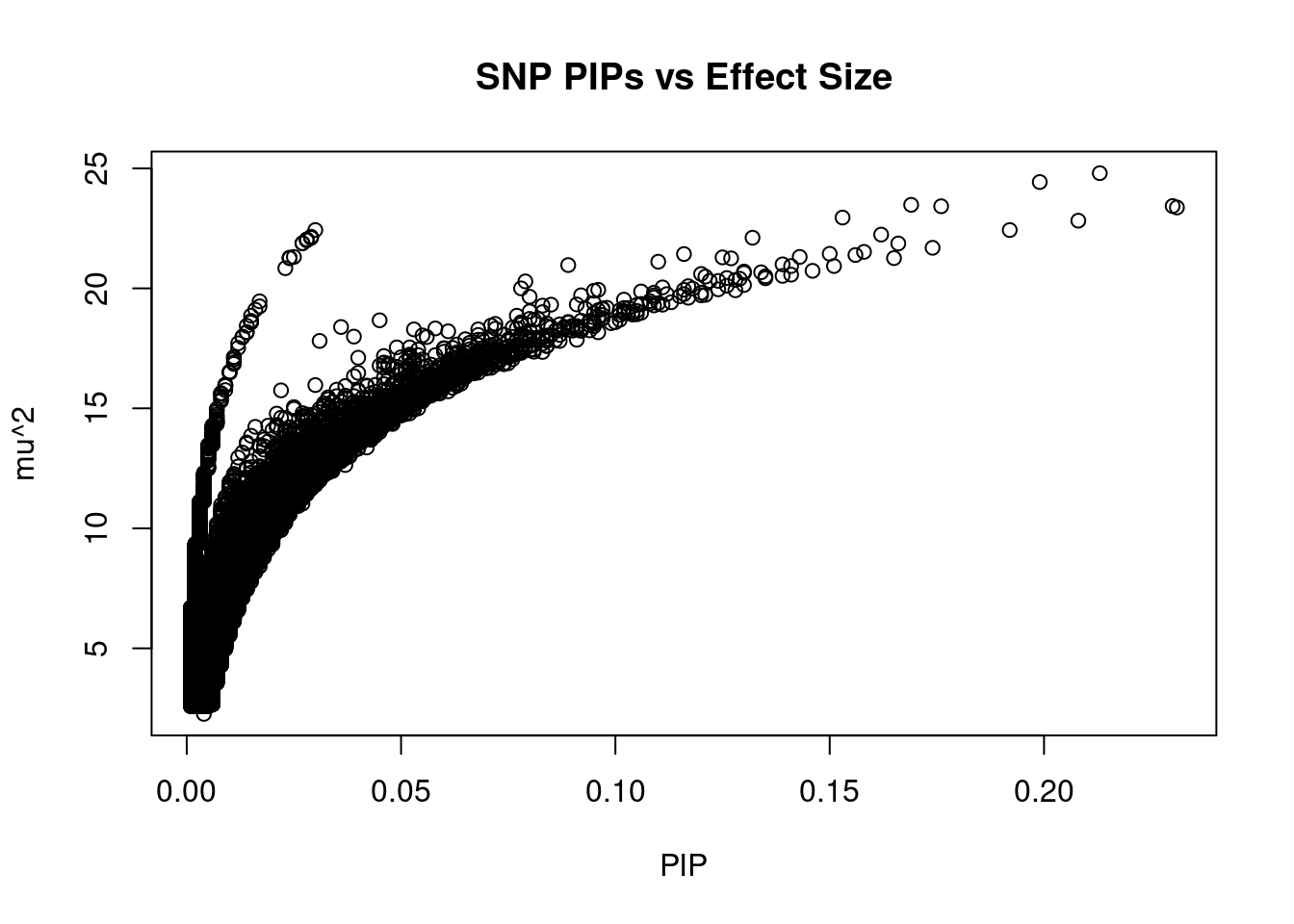
| Version | Author | Date |
|---|---|---|
| dfd2b5f | wesleycrouse | 2021-09-07 |
#SNPs with 50 largest effect sizes
head(ctwas_snp_res[order(-ctwas_snp_res$mu2),report_cols_snps],50) id region_tag susie_pip mu2 PVE z
761470 rs4968212 17_7 0.213 24.80 9.7e-05 -5.30
761480 rs62059839 17_7 0.199 24.43 8.9e-05 5.31
761458 rs9892862 17_7 0.169 23.48 7.3e-05 5.26
140037 rs75620930 3_1 0.230 23.43 9.9e-05 4.34
526099 rs551737161 10_16 0.176 23.42 7.6e-05 3.60
396298 rs62444120 7_28 0.231 23.37 9.9e-05 -4.35
761460 rs8073177 17_7 0.153 22.95 6.4e-05 5.21
476904 rs112371525 8_89 0.208 22.82 8.7e-05 4.23
333973 rs6913470 6_26 0.030 22.43 1.3e-05 4.92
679108 rs1959607 14_1 0.192 22.43 7.9e-05 4.21
70306 rs13429539 2_7 0.162 22.24 6.6e-05 -4.12
333928 rs915660 6_26 0.029 22.15 1.2e-05 4.89
333930 rs9357109 6_26 0.029 22.12 1.2e-05 4.88
761500 rs1641549 17_7 0.132 22.11 5.3e-05 -4.62
333967 rs7453202 6_26 0.029 22.10 1.2e-05 4.89
333971 rs6907451 6_26 0.028 22.04 1.1e-05 4.89
333957 rs28360066 6_26 0.028 22.02 1.1e-05 4.88
333964 rs9380223 6_26 0.028 22.00 1.1e-05 4.88
333953 rs9391819 6_26 0.027 21.88 1.1e-05 4.86
333960 rs9404983 6_26 0.027 21.88 1.1e-05 4.86
319306 rs13160700 5_104 0.166 21.87 6.7e-05 -4.06
813811 rs73000658 19_16 0.174 21.69 6.9e-05 -4.06
513886 rs10760461 9_65 0.158 21.52 6.2e-05 4.10
459963 rs553564201 8_57 0.150 21.44 5.9e-05 3.99
761464 rs11078694 17_7 0.116 21.43 4.6e-05 -5.05
417774 rs876169 7_68 0.156 21.39 6.1e-05 4.31
333947 rs28360062 6_26 0.025 21.31 9.6e-06 4.82
381048 rs113784847 7_3 0.143 21.31 5.6e-05 -4.04
39473 rs192164271 1_80 0.125 21.29 4.9e-05 3.94
333942 rs9378115 6_26 0.024 21.28 9.6e-06 4.81
165332 rs9813399 3_53 0.165 21.26 6.4e-05 4.14
333938 rs6899875 6_26 0.024 21.26 9.5e-06 4.81
333940 rs9357115 6_26 0.024 21.26 9.5e-06 4.81
138489 rs3791461 2_143 0.127 21.25 5.0e-05 -3.97
761474 rs149932962 17_7 0.110 21.11 4.3e-05 5.03
327669 rs192338591 6_13 0.139 21.00 5.4e-05 3.94
758122 rs11076740 16_54 0.089 20.97 3.4e-05 -3.98
272419 rs112157462 5_14 0.151 20.93 5.8e-05 3.98
806869 rs117700303 19_2 0.141 20.93 5.4e-05 -4.07
333976 rs17191237 6_26 0.023 20.84 8.6e-06 4.78
154020 rs76750610 3_28 0.146 20.73 5.5e-05 -3.93
742136 rs143894776 16_23 0.130 20.70 4.9e-05 -3.90
423042 rs9640863 7_80 0.134 20.67 5.1e-05 -3.96
96986 rs187600283 2_58 0.130 20.64 4.9e-05 3.88
642695 rs146568110 12_75 0.120 20.60 4.5e-05 3.84
2845 rs2274332 1_6 0.141 20.57 5.3e-05 -3.89
598854 rs73008432 11_70 0.139 20.52 5.2e-05 4.04
106803 rs62162215 2_78 0.135 20.50 5.1e-05 -3.94
182446 rs9823043 3_85 0.121 20.49 4.5e-05 -4.05
710597 rs76553406 15_9 0.126 20.43 4.7e-05 3.91SNPs with highest PVE
#SNPs with 50 highest pve
head(ctwas_snp_res[order(-ctwas_snp_res$PVE),report_cols_snps],50) id region_tag susie_pip mu2 PVE z
140037 rs75620930 3_1 0.230 23.43 9.9e-05 4.34
396298 rs62444120 7_28 0.231 23.37 9.9e-05 -4.35
761470 rs4968212 17_7 0.213 24.80 9.7e-05 -5.30
761480 rs62059839 17_7 0.199 24.43 8.9e-05 5.31
476904 rs112371525 8_89 0.208 22.82 8.7e-05 4.23
679108 rs1959607 14_1 0.192 22.43 7.9e-05 4.21
526099 rs551737161 10_16 0.176 23.42 7.6e-05 3.60
761458 rs9892862 17_7 0.169 23.48 7.3e-05 5.26
813811 rs73000658 19_16 0.174 21.69 6.9e-05 -4.06
319306 rs13160700 5_104 0.166 21.87 6.7e-05 -4.06
70306 rs13429539 2_7 0.162 22.24 6.6e-05 -4.12
165332 rs9813399 3_53 0.165 21.26 6.4e-05 4.14
761460 rs8073177 17_7 0.153 22.95 6.4e-05 5.21
513886 rs10760461 9_65 0.158 21.52 6.2e-05 4.10
417774 rs876169 7_68 0.156 21.39 6.1e-05 4.31
459963 rs553564201 8_57 0.150 21.44 5.9e-05 3.99
272419 rs112157462 5_14 0.151 20.93 5.8e-05 3.98
381048 rs113784847 7_3 0.143 21.31 5.6e-05 -4.04
154020 rs76750610 3_28 0.146 20.73 5.5e-05 -3.93
327669 rs192338591 6_13 0.139 21.00 5.4e-05 3.94
806869 rs117700303 19_2 0.141 20.93 5.4e-05 -4.07
2845 rs2274332 1_6 0.141 20.57 5.3e-05 -3.89
761500 rs1641549 17_7 0.132 22.11 5.3e-05 -4.62
598854 rs73008432 11_70 0.139 20.52 5.2e-05 4.04
106803 rs62162215 2_78 0.135 20.50 5.1e-05 -3.94
423042 rs9640863 7_80 0.134 20.67 5.1e-05 -3.96
64719 rs79505543 1_129 0.135 20.42 5.0e-05 3.85
138489 rs3791461 2_143 0.127 21.25 5.0e-05 -3.97
39473 rs192164271 1_80 0.125 21.29 4.9e-05 3.94
96986 rs187600283 2_58 0.130 20.64 4.9e-05 3.88
742136 rs143894776 16_23 0.130 20.70 4.9e-05 -3.90
24562 rs41284595 1_52 0.130 20.14 4.8e-05 -3.85
582710 rs140333597 11_37 0.129 20.40 4.8e-05 3.86
763904 rs58335060 17_13 0.128 20.35 4.8e-05 -4.13
56411 rs78763889 1_111 0.126 20.11 4.7e-05 -4.03
351728 rs78874260 6_54 0.128 19.92 4.7e-05 -3.79
710597 rs76553406 15_9 0.126 20.43 4.7e-05 3.91
761464 rs11078694 17_7 0.116 21.43 4.6e-05 -5.05
819786 rs544367902 19_27 0.124 20.31 4.6e-05 3.82
182446 rs9823043 3_85 0.121 20.49 4.5e-05 -4.05
368002 rs148062999 6_88 0.122 20.31 4.5e-05 3.84
642695 rs146568110 12_75 0.120 20.60 4.5e-05 3.84
645510 rs1696452 12_80 0.124 19.96 4.5e-05 3.78
362887 rs2350814 6_76 0.120 19.82 4.4e-05 3.91
764807 rs139270577 17_15 0.121 19.73 4.4e-05 -3.76
99704 rs3113783 2_65 0.120 19.71 4.3e-05 -3.99
257296 rs13121691 4_110 0.118 19.99 4.3e-05 -3.84
468856 rs149885805 8_74 0.117 20.09 4.3e-05 -3.82
761474 rs149932962 17_7 0.110 21.11 4.3e-05 5.03
73000 rs552412983 2_10 0.116 19.94 4.2e-05 4.04SNPs with largest z scores
#SNPs with 50 largest z scores
head(ctwas_snp_res[order(-abs(ctwas_snp_res$z)),report_cols_snps],50) id region_tag susie_pip mu2 PVE z
761480 rs62059839 17_7 0.199 24.43 8.9e-05 5.31
761470 rs4968212 17_7 0.213 24.80 9.7e-05 -5.30
761458 rs9892862 17_7 0.169 23.48 7.3e-05 5.26
761460 rs8073177 17_7 0.153 22.95 6.4e-05 5.21
761464 rs11078694 17_7 0.116 21.43 4.6e-05 -5.05
761474 rs149932962 17_7 0.110 21.11 4.3e-05 5.03
333973 rs6913470 6_26 0.030 22.43 1.3e-05 4.92
333928 rs915660 6_26 0.029 22.15 1.2e-05 4.89
333967 rs7453202 6_26 0.029 22.10 1.2e-05 4.89
333971 rs6907451 6_26 0.028 22.04 1.1e-05 4.89
333930 rs9357109 6_26 0.029 22.12 1.2e-05 4.88
333957 rs28360066 6_26 0.028 22.02 1.1e-05 4.88
333964 rs9380223 6_26 0.028 22.00 1.1e-05 4.88
333953 rs9391819 6_26 0.027 21.88 1.1e-05 4.86
333960 rs9404983 6_26 0.027 21.88 1.1e-05 4.86
333947 rs28360062 6_26 0.025 21.31 9.6e-06 4.82
333938 rs6899875 6_26 0.024 21.26 9.5e-06 4.81
333940 rs9357115 6_26 0.024 21.26 9.5e-06 4.81
333942 rs9378115 6_26 0.024 21.28 9.6e-06 4.81
333976 rs17191237 6_26 0.023 20.84 8.6e-06 4.78
760375 rs55884013 17_4 0.091 19.33 3.2e-05 -4.75
760372 rs55677157 17_4 0.081 18.69 2.8e-05 -4.69
760370 rs142108572 17_4 0.076 18.36 2.6e-05 -4.67
334039 rs28749561 6_26 0.017 19.46 6.2e-06 4.64
760373 rs11658808 17_4 0.082 18.72 2.8e-05 4.64
334022 rs28397294 6_26 0.017 19.25 5.9e-06 4.62
760371 rs2174830 17_4 0.078 18.49 2.7e-05 4.62
761500 rs1641549 17_7 0.132 22.11 5.3e-05 -4.62
334019 rs7745906 6_26 0.016 19.11 5.7e-06 4.60
760368 rs9904358 17_4 0.066 17.62 2.1e-05 4.58
760364 rs55780247 17_4 0.057 16.82 1.8e-05 -4.49
760367 rs4538065 17_4 0.053 16.40 1.6e-05 4.44
334078 rs35556213 6_26 0.015 18.60 5.0e-06 4.41
334079 rs35303934 6_26 0.014 18.44 4.8e-06 4.39
333956 rs28749557 6_26 0.013 17.99 4.3e-06 4.38
334087 rs35482069 6_26 0.014 18.18 4.5e-06 4.36
334088 rs34247821 6_26 0.014 18.16 4.5e-06 4.36
396298 rs62444120 7_28 0.231 23.37 9.9e-05 -4.35
140037 rs75620930 3_1 0.230 23.43 9.9e-05 4.34
760381 rs55999196 17_4 0.045 15.54 1.3e-05 -4.33
417774 rs876169 7_68 0.156 21.39 6.1e-05 4.31
572473 rs11031033 11_21 0.080 18.23 2.7e-05 -4.30
572475 rs10835646 11_21 0.079 18.15 2.6e-05 -4.30
333985 rs10947149 6_26 0.011 17.14 3.5e-06 4.27
155047 rs13092460 3_31 0.080 19.04 2.8e-05 -4.26
333986 rs9263947 6_26 0.015 18.64 5.1e-06 4.26
333935 rs9468882 6_26 0.011 17.12 3.5e-06 4.25
333983 rs34480332 6_26 0.011 17.04 3.4e-06 4.25
333988 rs3869109 6_26 0.015 18.58 5.0e-06 4.25
760363 rs12941694 17_4 0.044 15.44 1.3e-05 4.25Gene set enrichment for genes with PIP>0.8
#GO enrichment analysis
library(enrichR)Welcome to enrichR
Checking connection ... Enrichr ... Connection is Live!
FlyEnrichr ... Connection is available!
WormEnrichr ... Connection is available!
YeastEnrichr ... Connection is available!
FishEnrichr ... Connection is available!dbs <- c("GO_Biological_Process_2021", "GO_Cellular_Component_2021", "GO_Molecular_Function_2021")
genes <- ctwas_gene_res$genename[ctwas_gene_res$susie_pip>0.8]
#number of genes for gene set enrichment
length(genes)[1] 0if (length(genes)>0){
GO_enrichment <- enrichr(genes, dbs)
for (db in dbs){
print(db)
df <- GO_enrichment[[db]]
df <- df[df$Adjusted.P.value<0.05,c("Term", "Overlap", "Adjusted.P.value", "Genes")]
print(df)
}
#DisGeNET enrichment
# devtools::install_bitbucket("ibi_group/disgenet2r")
library(disgenet2r)
disgenet_api_key <- get_disgenet_api_key(
email = "wesleycrouse@gmail.com",
password = "uchicago1" )
Sys.setenv(DISGENET_API_KEY= disgenet_api_key)
res_enrich <-disease_enrichment(entities=genes, vocabulary = "HGNC",
database = "CURATED" )
df <- res_enrich@qresult[1:10, c("Description", "FDR", "Ratio", "BgRatio")]
print(df)
#WebGestalt enrichment
library(WebGestaltR)
background <- ctwas_gene_res$genename
#listGeneSet()
databases <- c("pathway_KEGG", "disease_GLAD4U", "disease_OMIM")
enrichResult <- WebGestaltR(enrichMethod="ORA", organism="hsapiens",
interestGene=genes, referenceGene=background,
enrichDatabase=databases, interestGeneType="genesymbol",
referenceGeneType="genesymbol", isOutput=F)
print(enrichResult[,c("description", "size", "overlap", "FDR", "database", "userId")])
}
sessionInfo()R version 3.6.1 (2019-07-05)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: Scientific Linux 7.4 (Nitrogen)
Matrix products: default
BLAS/LAPACK: /software/openblas-0.2.19-el7-x86_64/lib/libopenblas_haswellp-r0.2.19.so
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
[3] LC_TIME=en_US.UTF-8 LC_COLLATE=en_US.UTF-8
[5] LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=en_US.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] enrichR_3.0 cowplot_1.0.0 ggplot2_3.3.3
loaded via a namespace (and not attached):
[1] Biobase_2.44.0 httr_1.4.1
[3] bit64_4.0.5 assertthat_0.2.1
[5] stats4_3.6.1 blob_1.2.1
[7] BSgenome_1.52.0 GenomeInfoDbData_1.2.1
[9] Rsamtools_2.0.0 yaml_2.2.0
[11] progress_1.2.2 pillar_1.6.1
[13] RSQLite_2.2.7 lattice_0.20-38
[15] glue_1.4.2 digest_0.6.20
[17] GenomicRanges_1.36.0 promises_1.0.1
[19] XVector_0.24.0 colorspace_1.4-1
[21] htmltools_0.3.6 httpuv_1.5.1
[23] Matrix_1.2-18 XML_3.98-1.20
[25] pkgconfig_2.0.3 biomaRt_2.40.1
[27] zlibbioc_1.30.0 purrr_0.3.4
[29] scales_1.1.0 whisker_0.3-2
[31] later_0.8.0 BiocParallel_1.18.0
[33] git2r_0.26.1 tibble_3.1.2
[35] farver_2.1.0 generics_0.0.2
[37] IRanges_2.18.1 ellipsis_0.3.2
[39] withr_2.4.1 cachem_1.0.5
[41] SummarizedExperiment_1.14.1 GenomicFeatures_1.36.3
[43] BiocGenerics_0.30.0 magrittr_2.0.1
[45] crayon_1.4.1 memoise_2.0.0
[47] evaluate_0.14 fs_1.3.1
[49] fansi_0.5.0 tools_3.6.1
[51] data.table_1.14.0 prettyunits_1.0.2
[53] hms_1.1.0 lifecycle_1.0.0
[55] matrixStats_0.57.0 stringr_1.4.0
[57] S4Vectors_0.22.1 munsell_0.5.0
[59] DelayedArray_0.10.0 AnnotationDbi_1.46.0
[61] Biostrings_2.52.0 compiler_3.6.1
[63] GenomeInfoDb_1.20.0 rlang_0.4.11
[65] grid_3.6.1 RCurl_1.98-1.1
[67] rjson_0.2.20 VariantAnnotation_1.30.1
[69] labeling_0.3 bitops_1.0-6
[71] rmarkdown_1.13 gtable_0.3.0
[73] curl_3.3 DBI_1.1.1
[75] R6_2.5.0 GenomicAlignments_1.20.1
[77] dplyr_1.0.7 knitr_1.23
[79] rtracklayer_1.44.0 utf8_1.2.1
[81] fastmap_1.1.0 bit_4.0.4
[83] workflowr_1.6.2 rprojroot_2.0.2
[85] stringi_1.4.3 parallel_3.6.1
[87] Rcpp_1.0.6 vctrs_0.3.8
[89] tidyselect_1.1.0 xfun_0.8