Lipoprotein A (quantile) - Liver
wesleycrouse
2021-09-09
Last updated: 2021-09-09
Checks: 6 1
Knit directory: ctwas_applied/
This reproducible R Markdown analysis was created with workflowr (version 1.6.2). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
The R Markdown file has unstaged changes. To know which version of the R Markdown file created these results, you’ll want to first commit it to the Git repo. If you’re still working on the analysis, you can ignore this warning. When you’re finished, you can run wflow_publish to commit the R Markdown file and build the HTML.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20210726) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version 59e5f4d. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
Unstaged changes:
Modified: analysis/ukb-d-30500_irnt_Liver.Rmd
Modified: analysis/ukb-d-30600_irnt_Liver.Rmd
Modified: analysis/ukb-d-30610_irnt_Liver.Rmd
Modified: analysis/ukb-d-30620_irnt_Liver.Rmd
Modified: analysis/ukb-d-30630_irnt_Liver.Rmd
Modified: analysis/ukb-d-30640_irnt_Liver.Rmd
Modified: analysis/ukb-d-30650_irnt_Liver.Rmd
Modified: analysis/ukb-d-30660_irnt_Liver.Rmd
Modified: analysis/ukb-d-30670_irnt_Liver.Rmd
Modified: analysis/ukb-d-30680_irnt_Liver.Rmd
Modified: analysis/ukb-d-30690_irnt_Liver.Rmd
Modified: analysis/ukb-d-30700_irnt_Liver.Rmd
Modified: analysis/ukb-d-30710_irnt_Liver.Rmd
Modified: analysis/ukb-d-30720_irnt_Liver.Rmd
Modified: analysis/ukb-d-30730_irnt_Liver.Rmd
Modified: analysis/ukb-d-30740_irnt_Liver.Rmd
Modified: analysis/ukb-d-30750_irnt_Liver.Rmd
Modified: analysis/ukb-d-30760_irnt_Liver.Rmd
Modified: analysis/ukb-d-30770_irnt_Liver.Rmd
Modified: analysis/ukb-d-30780_irnt_Liver.Rmd
Modified: analysis/ukb-d-30790_irnt_Liver.Rmd
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were made to the R Markdown (analysis/ukb-d-30790_irnt_Liver.Rmd) and HTML (docs/ukb-d-30790_irnt_Liver.html) files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view the files as they were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | cbf7408 | wesleycrouse | 2021-09-08 | adding enrichment to reports |
| html | cbf7408 | wesleycrouse | 2021-09-08 | adding enrichment to reports |
| Rmd | 4970e3e | wesleycrouse | 2021-09-08 | updating reports |
| html | 4970e3e | wesleycrouse | 2021-09-08 | updating reports |
| Rmd | 627a4e1 | wesleycrouse | 2021-09-07 | adding heritability |
| Rmd | dfd2b5f | wesleycrouse | 2021-09-07 | regenerating reports |
| html | dfd2b5f | wesleycrouse | 2021-09-07 | regenerating reports |
| Rmd | 61b53b3 | wesleycrouse | 2021-09-06 | updated PVE calculation |
| html | 61b53b3 | wesleycrouse | 2021-09-06 | updated PVE calculation |
| Rmd | 837dd01 | wesleycrouse | 2021-09-01 | adding additional fixedsigma report |
| Rmd | d0a5417 | wesleycrouse | 2021-08-30 | adding new reports to the index |
| Rmd | 0922de7 | wesleycrouse | 2021-08-18 | updating all reports with locus plots |
| html | 0922de7 | wesleycrouse | 2021-08-18 | updating all reports with locus plots |
| html | 1c62980 | wesleycrouse | 2021-08-11 | Updating reports |
| Rmd | 5981e80 | wesleycrouse | 2021-08-11 | Adding more reports |
| html | 5981e80 | wesleycrouse | 2021-08-11 | Adding more reports |
| Rmd | da9f015 | wesleycrouse | 2021-08-07 | adding more reports |
| html | da9f015 | wesleycrouse | 2021-08-07 | adding more reports |
Overview
These are the results of a ctwas analysis of the UK Biobank trait Lipoprotein A (quantile) using Liver gene weights.
The GWAS was conducted by the Neale Lab, and the biomarker traits we analyzed are discussed here. Summary statistics were obtained from IEU OpenGWAS using GWAS ID: ukb-d-30790_irnt. Results were obtained from from IEU rather than Neale Lab because they are in a standardard format (GWAS VCF). Note that 3 of the 34 biomarker traits were not available from IEU and were excluded from analysis.
The weights are mashr GTEx v8 models on Liver eQTL obtained from PredictDB. We performed a full harmonization of the variants, including recovering strand ambiguous variants. This procedure is discussed in a separate document. (TO-DO: add report that describes harmonization)
LD matrices were computed from a 10% subset of Neale lab subjects. Subjects were matched using the plate and well information from genotyping. We included only biallelic variants with MAF>0.01 in the original Neale Lab GWAS. (TO-DO: add more details [number of subjects, variants, etc])
Weight QC
TO-DO: add enhanced QC reporting (total number of weights, why each variant was missing for all genes)
qclist_all <- list()
qc_files <- paste0(results_dir, "/", list.files(results_dir, pattern="exprqc.Rd"))
for (i in 1:length(qc_files)){
load(qc_files[i])
chr <- unlist(strsplit(rev(unlist(strsplit(qc_files[i], "_")))[1], "[.]"))[1]
qclist_all[[chr]] <- cbind(do.call(rbind, lapply(qclist,unlist)), as.numeric(substring(chr,4)))
}
qclist_all <- data.frame(do.call(rbind, qclist_all))
colnames(qclist_all)[ncol(qclist_all)] <- "chr"
rm(qclist, wgtlist, z_gene_chr)
#number of imputed weights
nrow(qclist_all)[1] 10901#number of imputed weights by chromosome
table(qclist_all$chr)
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15
1070 768 652 417 494 611 548 408 405 434 634 629 195 365 354
16 17 18 19 20 21 22
526 663 160 859 306 114 289 #proportion of imputed weights without missing variants
mean(qclist_all$nmiss==0)[1] 0.8366205Load ctwas results
#load ctwas results
ctwas_res <- data.table::fread(paste0(results_dir, "/", analysis_id, "_ctwas.susieIrss.txt"))
#make unique identifier for regions
ctwas_res$region_tag <- paste(ctwas_res$region_tag1, ctwas_res$region_tag2, sep="_")
#compute PVE for each gene/SNP
ctwas_res$PVE = ctwas_res$susie_pip*ctwas_res$mu2/sample_size #check PVE calculation
#separate gene and SNP results
ctwas_gene_res <- ctwas_res[ctwas_res$type == "gene", ]
ctwas_gene_res <- data.frame(ctwas_gene_res)
ctwas_snp_res <- ctwas_res[ctwas_res$type == "SNP", ]
ctwas_snp_res <- data.frame(ctwas_snp_res)
#add gene information to results
sqlite <- RSQLite::dbDriver("SQLite")
db = RSQLite::dbConnect(sqlite, paste0("/project2/compbio/predictdb/mashr_models/mashr_", weight, ".db"))
query <- function(...) RSQLite::dbGetQuery(db, ...)
gene_info <- query("select gene, genename from extra")
gene_info <- query("select gene, genename, gene_type from extra")
RSQLite::dbDisconnect(db)
ctwas_gene_res <- cbind(ctwas_gene_res, gene_info[sapply(ctwas_gene_res$id, match, gene_info$gene), c("genename", "gene_type")])
#add z score to results
load(paste0(results_dir, "/", analysis_id, "_expr_z_gene.Rd"))
ctwas_gene_res$z <- z_gene[ctwas_gene_res$id,]$z
#load(paste0(results_dir, "/", analysis_id, "_expr_z_snp.Rd")) #for new version, stored after harmonization
z_snp <- readRDS(paste0(results_dir, "/", trait_id, ".RDS")) #for old version, unharmonized
z_snp <- z_snp[z_snp$id %in% ctwas_snp_res$id,] #subset snps to those included in analysis, note some are duplicated, need to match which allele was used
ctwas_snp_res$z <- z_snp$z[match(ctwas_snp_res$id, z_snp$id)] #for duplicated snps, this arbitrarily uses the first allele
ctwas_snp_res$z_flag <- as.numeric(ctwas_snp_res$id %in% z_snp$id[duplicated(z_snp$id)]) #mark the unclear z scores, flag=1
#formatting and rounding for tables
ctwas_gene_res$z <- round(ctwas_gene_res$z,2)
ctwas_snp_res$z <- round(ctwas_snp_res$z,2)
ctwas_gene_res$susie_pip <- round(ctwas_gene_res$susie_pip,3)
ctwas_snp_res$susie_pip <- round(ctwas_snp_res$susie_pip,3)
ctwas_gene_res$mu2 <- round(ctwas_gene_res$mu2,2)
ctwas_snp_res$mu2 <- round(ctwas_snp_res$mu2,2)
ctwas_gene_res$PVE <- signif(ctwas_gene_res$PVE, 2)
ctwas_snp_res$PVE <- signif(ctwas_snp_res$PVE, 2)
#merge gene and snp results with added information
ctwas_gene_res$z_flag=NA
ctwas_snp_res$genename=NA
ctwas_snp_res$gene_type=NA
ctwas_res <- rbind(ctwas_gene_res,
ctwas_snp_res[,colnames(ctwas_gene_res)])
#store columns to report
report_cols <- colnames(ctwas_gene_res)[!(colnames(ctwas_gene_res) %in% c("type", "region_tag1", "region_tag2", "cs_index", "gene_type", "z_flag", "id", "chrom", "pos"))]
first_cols <- c("genename", "region_tag")
report_cols <- c(first_cols, report_cols[!(report_cols %in% first_cols)])
report_cols_snps <- c("id", report_cols[-1])
#get number of SNPs from s1 results; adjust for thin
ctwas_res_s1 <- data.table::fread(paste0(results_dir, "/", analysis_id, "_ctwas.s1.susieIrss.txt"))
n_snps <- sum(ctwas_res_s1$type=="SNP")/thin
rm(ctwas_res_s1)Check convergence of parameters
library(ggplot2)
library(cowplot)
********************************************************Note: As of version 1.0.0, cowplot does not change the default ggplot2 theme anymore. To recover the previous behavior, execute:
theme_set(theme_cowplot())********************************************************load(paste0(results_dir, "/", analysis_id, "_ctwas.s2.susieIrssres.Rd"))
df <- data.frame(niter = rep(1:ncol(group_prior_rec), 2),
value = c(group_prior_rec[1,], group_prior_rec[2,]),
group = rep(c("Gene", "SNP"), each = ncol(group_prior_rec)))
df$group <- as.factor(df$group)
df$value[df$group=="SNP"] <- df$value[df$group=="SNP"]*thin #adjust parameter to account for thin argument
p_pi <- ggplot(df, aes(x=niter, y=value, group=group)) +
geom_line(aes(color=group)) +
geom_point(aes(color=group)) +
xlab("Iteration") + ylab(bquote(pi)) +
ggtitle("Prior mean") +
theme_cowplot()
df <- data.frame(niter = rep(1:ncol(group_prior_var_rec), 2),
value = c(group_prior_var_rec[1,], group_prior_var_rec[2,]),
group = rep(c("Gene", "SNP"), each = ncol(group_prior_var_rec)))
df$group <- as.factor(df$group)
p_sigma2 <- ggplot(df, aes(x=niter, y=value, group=group)) +
geom_line(aes(color=group)) +
geom_point(aes(color=group)) +
xlab("Iteration") + ylab(bquote(sigma^2)) +
ggtitle("Prior variance") +
theme_cowplot()
plot_grid(p_pi, p_sigma2)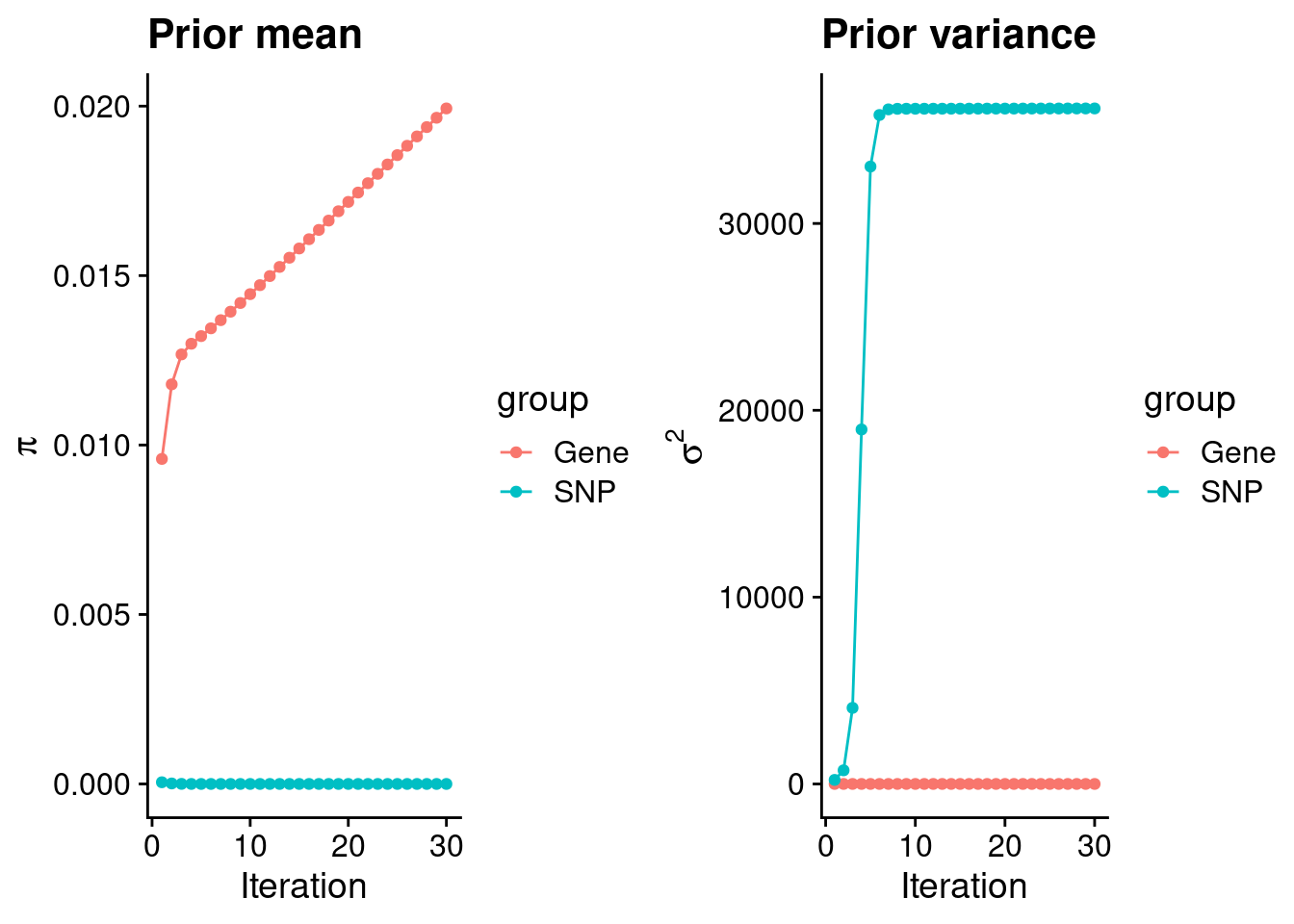
| Version | Author | Date |
|---|---|---|
| dfd2b5f | wesleycrouse | 2021-09-07 |
#estimated group prior
estimated_group_prior <- group_prior_rec[,ncol(group_prior_rec)]
names(estimated_group_prior) <- c("gene", "snp")
estimated_group_prior["snp"] <- estimated_group_prior["snp"]*thin #adjust parameter to account for thin argument
print(estimated_group_prior) gene snp
1.993189e-02 2.995612e-07 #estimated group prior variance
estimated_group_prior_var <- group_prior_var_rec[,ncol(group_prior_var_rec)]
names(estimated_group_prior_var) <- c("gene", "snp")
print(estimated_group_prior_var) gene snp
1.624517 36154.672592 #report sample size
print(sample_size)[1] 273896#report group size
group_size <- c(nrow(ctwas_gene_res), n_snps)
print(group_size)[1] 10901 8697330#estimated group PVE
estimated_group_pve <- estimated_group_prior_var*estimated_group_prior*group_size/sample_size #check PVE calculation
names(estimated_group_pve) <- c("gene", "snp")
print(estimated_group_pve) gene snp
0.001288705 0.343914326 #compare sum(PIP*mu2/sample_size) with above PVE calculation
c(sum(ctwas_gene_res$PVE),sum(ctwas_snp_res$PVE))[1] 0.0200531 0.4610330Genes with highest PIPs
#distribution of PIPs
hist(ctwas_gene_res$susie_pip, xlim=c(0,1), main="Distribution of Gene PIPs")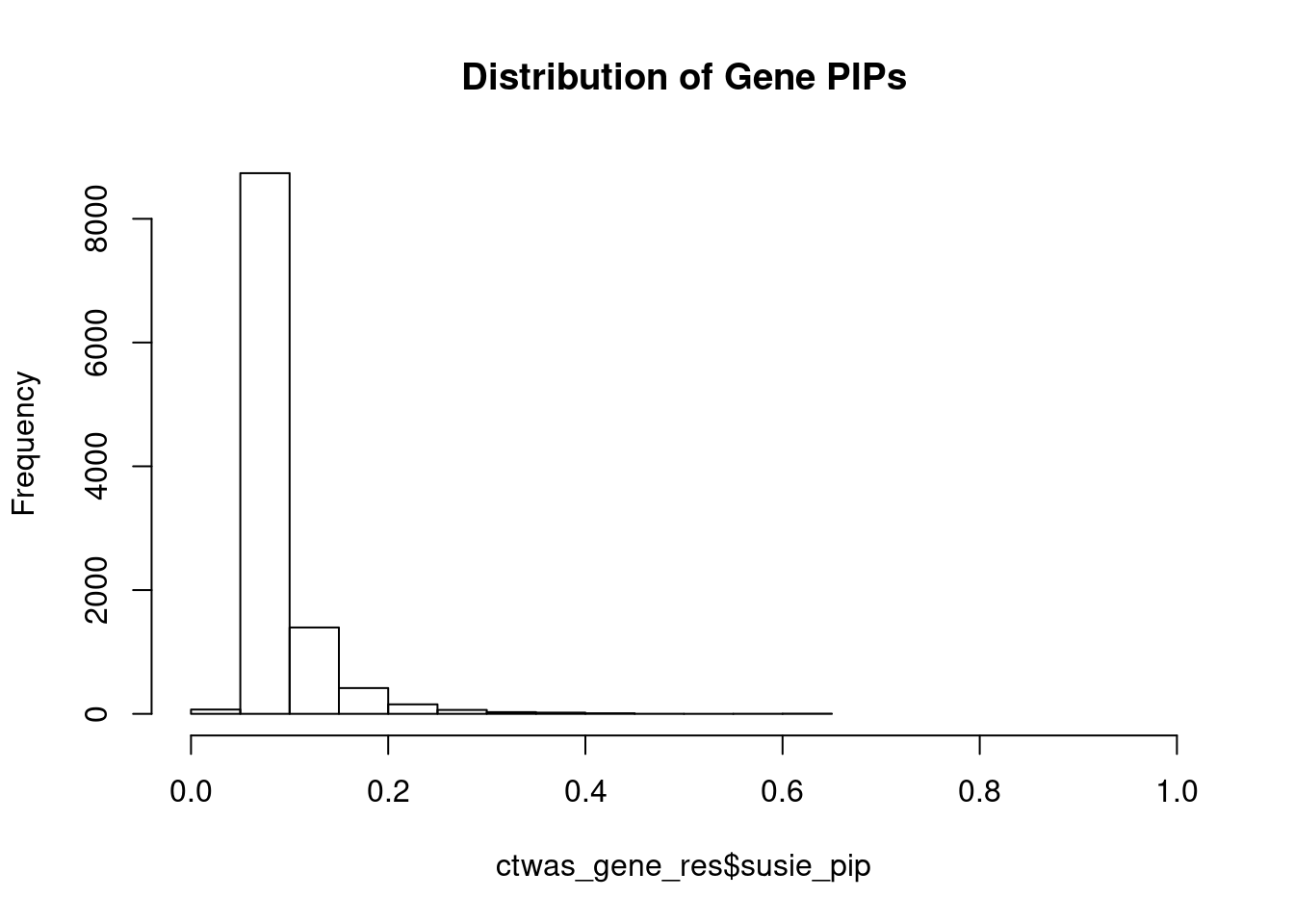
| Version | Author | Date |
|---|---|---|
| dfd2b5f | wesleycrouse | 2021-09-07 |
#genes with PIP>0.8 or 20 highest PIPs
head(ctwas_gene_res[order(-ctwas_gene_res$susie_pip),report_cols], max(sum(ctwas_gene_res$susie_pip>0.8), 20)) genename region_tag susie_pip mu2 PVE z
7656 CATSPER2 15_16 0.629 20.97 4.8e-05 -4.75
4286 PRMT7 16_36 0.619 19.88 4.5e-05 4.56
12008 HPR 16_38 0.606 20.12 4.5e-05 -4.26
8865 FUT2 19_33 0.582 18.95 4.0e-05 -4.13
10585 HSD17B8 6_27 0.484 18.24 3.2e-05 3.74
4435 PSRC1 1_67 0.450 18.16 3.0e-05 -4.01
5648 ABHD10 3_70 0.450 16.77 2.8e-05 -3.42
6118 TEX9 15_24 0.449 17.04 2.8e-05 -3.45
8882 RHOG 11_3 0.435 17.11 2.7e-05 -3.43
5732 COMMD10 5_69 0.422 16.69 2.6e-05 3.35
12032 U91319.1 16_14 0.417 16.66 2.5e-05 3.34
8629 PHLDA3 1_101 0.413 16.39 2.5e-05 -3.30
4859 RNF121 11_40 0.405 16.00 2.4e-05 3.23
9463 EMILIN3 20_25 0.404 16.83 2.5e-05 3.45
6168 GEMIN6 2_24 0.403 16.30 2.4e-05 -3.27
9127 TSHZ1 18_44 0.400 16.26 2.4e-05 -3.26
2888 ITGB6 2_96 0.396 16.09 2.3e-05 -3.23
3272 EIF2B2 14_34 0.393 15.67 2.2e-05 -3.24
6724 TNFRSF13C 22_17 0.392 15.63 2.2e-05 3.12
463 HHAT 1_106 0.379 15.83 2.2e-05 3.17Genes with largest effect sizes
#plot PIP vs effect size
plot(ctwas_gene_res$susie_pip, ctwas_gene_res$mu2, xlab="PIP", ylab="mu^2", main="Gene PIPs vs Effect Size")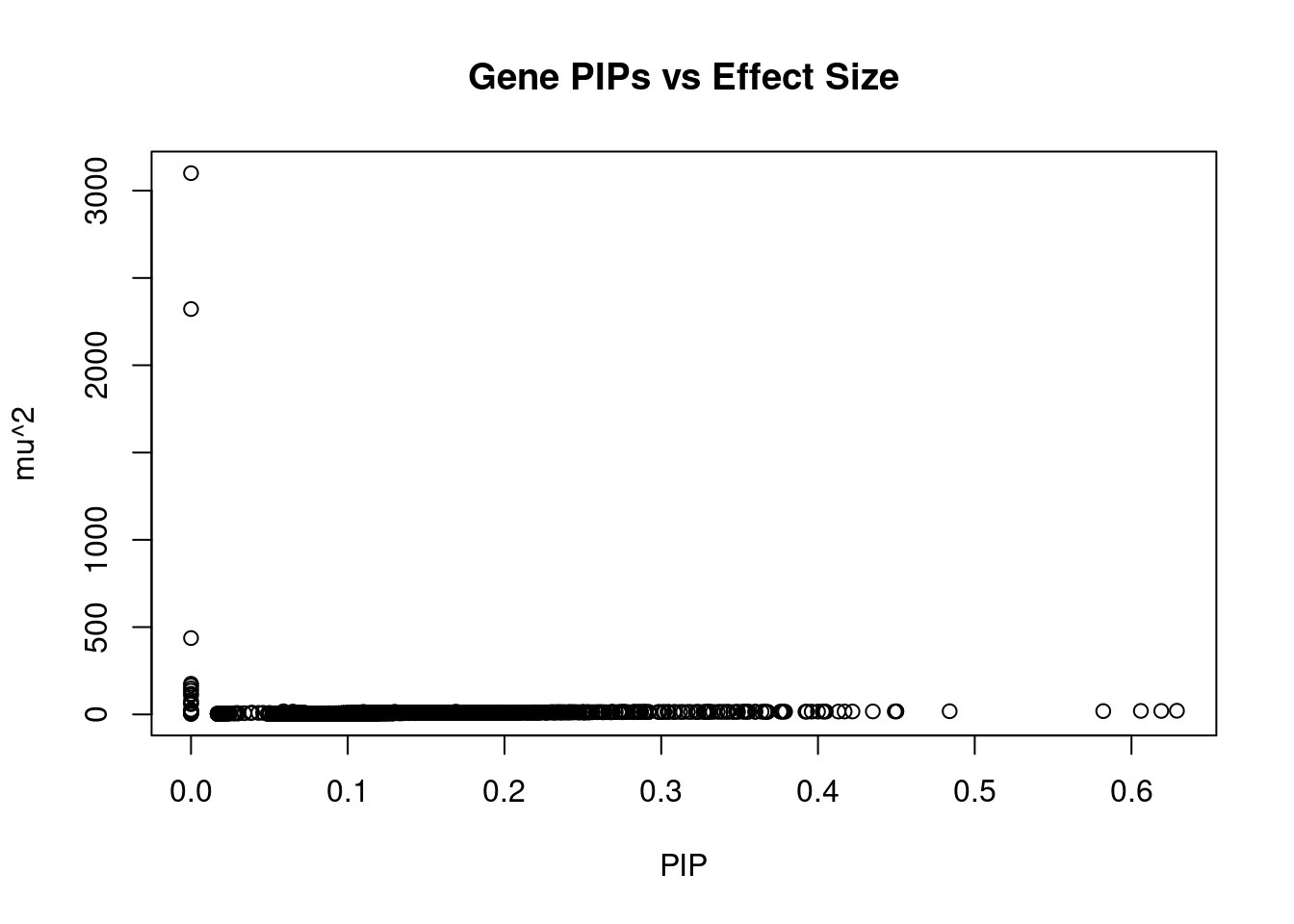
| Version | Author | Date |
|---|---|---|
| dfd2b5f | wesleycrouse | 2021-09-07 |
#genes with 20 largest effect sizes
head(ctwas_gene_res[order(-ctwas_gene_res$mu2),report_cols],20) genename region_tag susie_pip mu2 PVE z
10435 LPA 6_104 0.000 3100.25 0.0e+00 60.08
5799 SLC22A3 6_104 0.000 2321.99 0.0e+00 -80.70
3449 PLG 6_104 0.000 437.35 0.0e+00 48.04
5796 WTAP 6_103 0.000 175.68 0.0e+00 7.02
11043 RP1-81D8.3 6_104 0.000 165.64 0.0e+00 -62.47
1074 MAP3K4 6_104 0.000 148.80 0.0e+00 24.06
11375 RP3-393E18.2 6_103 0.000 135.84 0.0e+00 -13.28
2628 SOD2 6_103 0.000 118.12 0.0e+00 -7.20
2629 MRPL18 6_103 0.000 109.26 0.0e+00 -6.69
5795 PNLDC1 6_103 0.000 79.01 0.0e+00 4.09
5793 DYNLT1 6_103 0.000 66.15 0.0e+00 -6.33
7349 SYTL3 6_103 0.000 58.48 0.0e+00 5.73
4070 RSPH3 6_103 0.000 26.92 0.0e+00 3.37
7656 CATSPER2 15_16 0.629 20.97 4.8e-05 -4.75
10185 IGF2R 6_103 0.000 20.68 0.0e+00 -8.46
12008 HPR 16_38 0.606 20.12 4.5e-05 -4.26
4286 PRMT7 16_36 0.619 19.88 4.5e-05 4.56
8865 FUT2 19_33 0.582 18.95 4.0e-05 -4.13
10585 HSD17B8 6_27 0.484 18.24 3.2e-05 3.74
4435 PSRC1 1_67 0.450 18.16 3.0e-05 -4.01Genes with highest PVE
#genes with 20 highest pve
head(ctwas_gene_res[order(-ctwas_gene_res$PVE),report_cols],20) genename region_tag susie_pip mu2 PVE z
7656 CATSPER2 15_16 0.629 20.97 4.8e-05 -4.75
4286 PRMT7 16_36 0.619 19.88 4.5e-05 4.56
12008 HPR 16_38 0.606 20.12 4.5e-05 -4.26
8865 FUT2 19_33 0.582 18.95 4.0e-05 -4.13
10585 HSD17B8 6_27 0.484 18.24 3.2e-05 3.74
4435 PSRC1 1_67 0.450 18.16 3.0e-05 -4.01
5648 ABHD10 3_70 0.450 16.77 2.8e-05 -3.42
6118 TEX9 15_24 0.449 17.04 2.8e-05 -3.45
8882 RHOG 11_3 0.435 17.11 2.7e-05 -3.43
5732 COMMD10 5_69 0.422 16.69 2.6e-05 3.35
8629 PHLDA3 1_101 0.413 16.39 2.5e-05 -3.30
12032 U91319.1 16_14 0.417 16.66 2.5e-05 3.34
9463 EMILIN3 20_25 0.404 16.83 2.5e-05 3.45
6168 GEMIN6 2_24 0.403 16.30 2.4e-05 -3.27
4859 RNF121 11_40 0.405 16.00 2.4e-05 3.23
9127 TSHZ1 18_44 0.400 16.26 2.4e-05 -3.26
2888 ITGB6 2_96 0.396 16.09 2.3e-05 -3.23
463 HHAT 1_106 0.379 15.83 2.2e-05 3.17
4653 SERPINE2 2_132 0.378 15.86 2.2e-05 -3.16
2240 SEC23IP 10_74 0.377 16.00 2.2e-05 3.70Genes with largest z scores
#genes with 20 largest z scores
head(ctwas_gene_res[order(-abs(ctwas_gene_res$z)),report_cols],20) genename region_tag susie_pip mu2 PVE z
5799 SLC22A3 6_104 0.000 2321.99 0.0e+00 -80.70
11043 RP1-81D8.3 6_104 0.000 165.64 0.0e+00 -62.47
10435 LPA 6_104 0.000 3100.25 0.0e+00 60.08
3449 PLG 6_104 0.000 437.35 0.0e+00 48.04
1074 MAP3K4 6_104 0.000 148.80 0.0e+00 24.06
11375 RP3-393E18.2 6_103 0.000 135.84 0.0e+00 -13.28
10185 IGF2R 6_103 0.000 20.68 0.0e+00 -8.46
2628 SOD2 6_103 0.000 118.12 0.0e+00 -7.20
5796 WTAP 6_103 0.000 175.68 0.0e+00 7.02
2629 MRPL18 6_103 0.000 109.26 0.0e+00 -6.69
5793 DYNLT1 6_103 0.000 66.15 0.0e+00 -6.33
5240 NLRC5 16_31 0.059 17.83 3.8e-06 6.20
7349 SYTL3 6_103 0.000 58.48 0.0e+00 5.73
1120 CETP 16_31 0.065 16.09 3.8e-06 5.71
7656 CATSPER2 15_16 0.629 20.97 4.8e-05 -4.75
4286 PRMT7 16_36 0.619 19.88 4.5e-05 4.56
12008 HPR 16_38 0.606 20.12 4.5e-05 -4.26
8865 FUT2 19_33 0.582 18.95 4.0e-05 -4.13
5795 PNLDC1 6_103 0.000 79.01 0.0e+00 4.09
4435 PSRC1 1_67 0.450 18.16 3.0e-05 -4.01Comparing z scores and PIPs
#set nominal signifiance threshold for z scores
alpha <- 0.05
#bonferroni adjusted threshold for z scores
sig_thresh <- qnorm(1-(alpha/nrow(ctwas_gene_res)/2), lower=T)
#Q-Q plot for z scores
obs_z <- ctwas_gene_res$z[order(ctwas_gene_res$z)]
exp_z <- qnorm((1:nrow(ctwas_gene_res))/nrow(ctwas_gene_res))
plot(exp_z, obs_z, xlab="Expected z", ylab="Observed z", main="Gene z score Q-Q plot")
abline(a=0,b=1)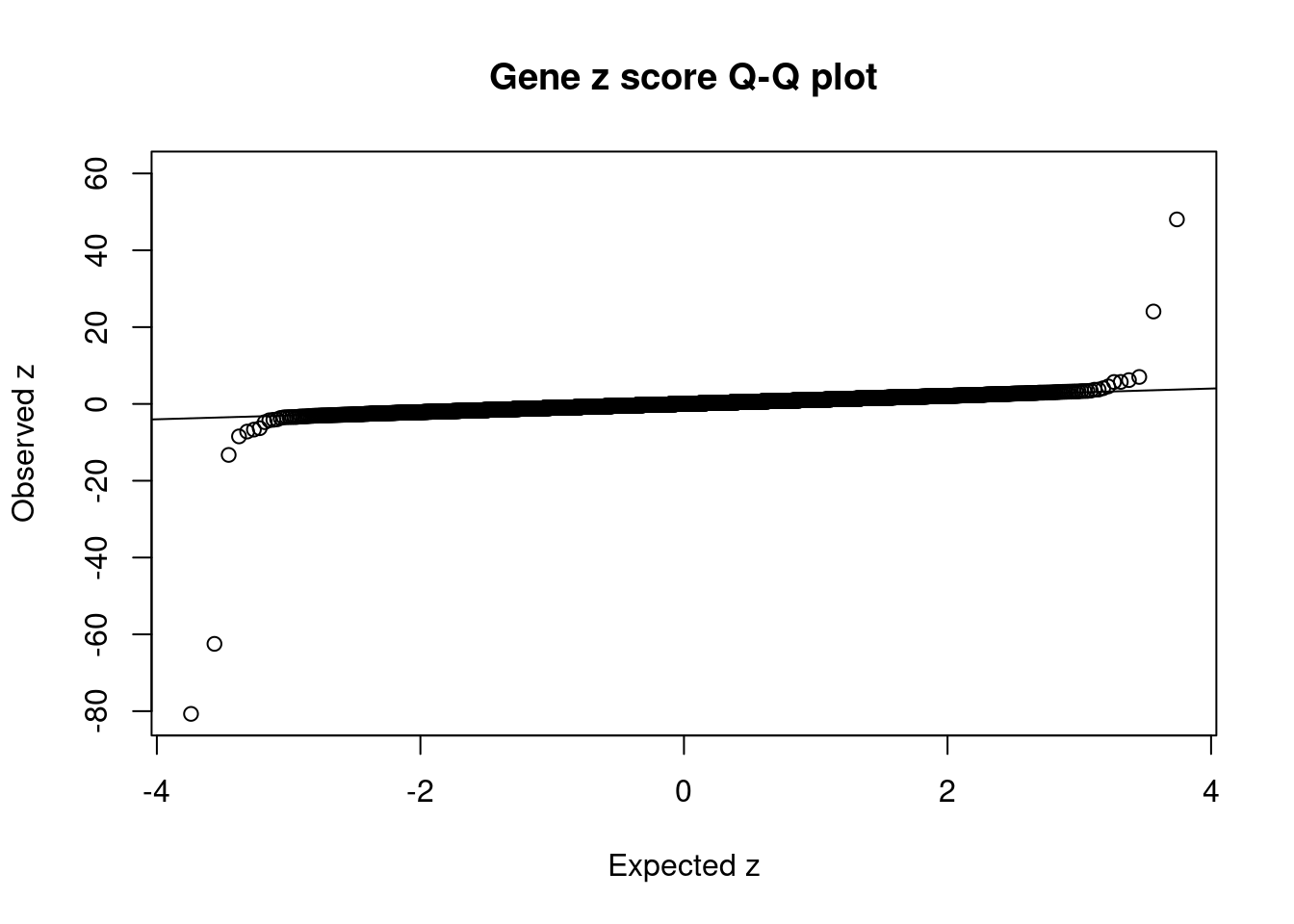
| Version | Author | Date |
|---|---|---|
| dfd2b5f | wesleycrouse | 2021-09-07 |
#plot z score vs PIP
plot(abs(ctwas_gene_res$z), ctwas_gene_res$susie_pip, xlab="abs(z)", ylab="PIP")
abline(v=sig_thresh, col="red", lty=2)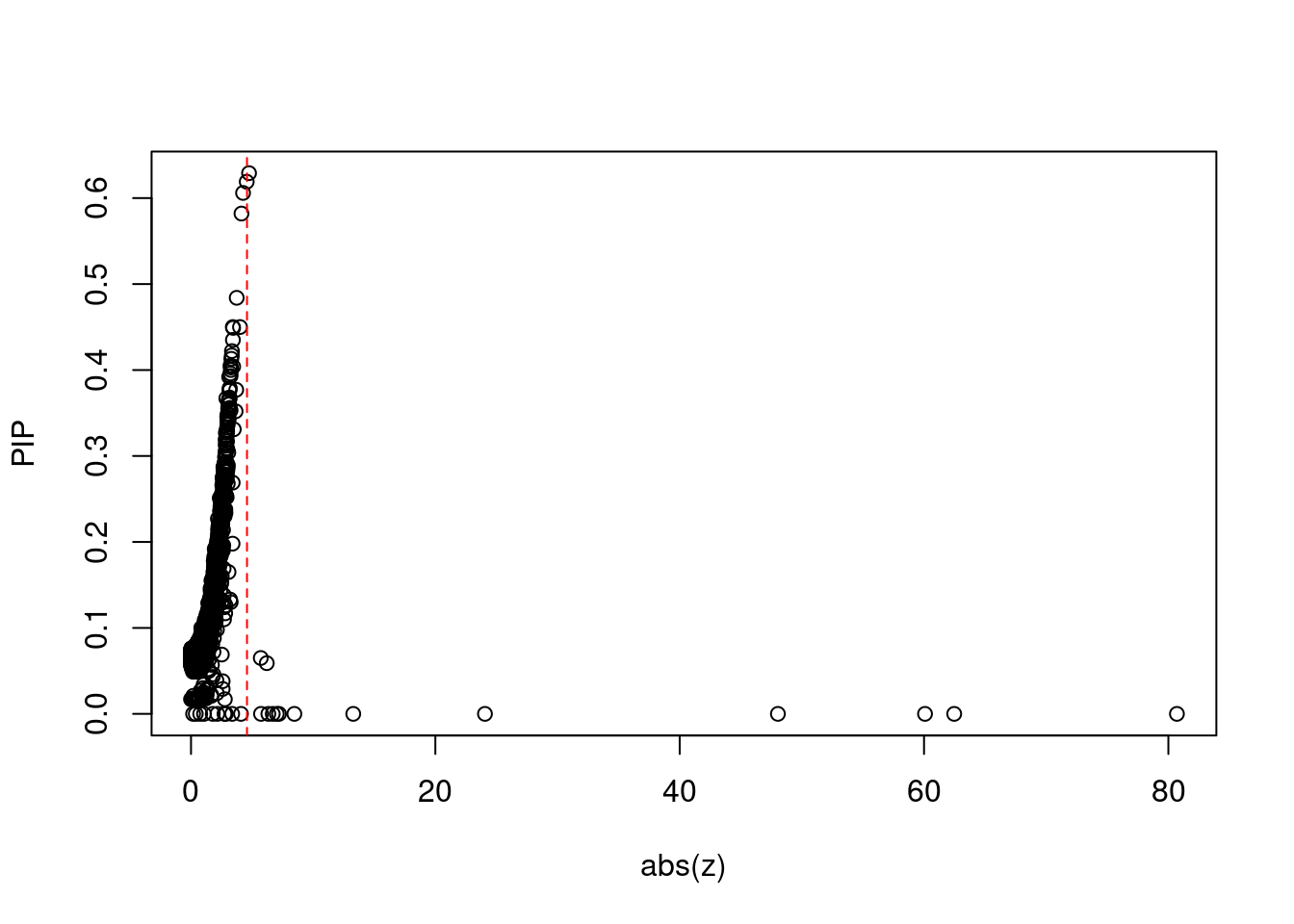
| Version | Author | Date |
|---|---|---|
| dfd2b5f | wesleycrouse | 2021-09-07 |
#proportion of significant z scores
mean(abs(ctwas_gene_res$z) > sig_thresh)[1] 0.001376021#genes with most significant z scores
head(ctwas_gene_res[order(-abs(ctwas_gene_res$z)),report_cols],20) genename region_tag susie_pip mu2 PVE z
5799 SLC22A3 6_104 0.000 2321.99 0.0e+00 -80.70
11043 RP1-81D8.3 6_104 0.000 165.64 0.0e+00 -62.47
10435 LPA 6_104 0.000 3100.25 0.0e+00 60.08
3449 PLG 6_104 0.000 437.35 0.0e+00 48.04
1074 MAP3K4 6_104 0.000 148.80 0.0e+00 24.06
11375 RP3-393E18.2 6_103 0.000 135.84 0.0e+00 -13.28
10185 IGF2R 6_103 0.000 20.68 0.0e+00 -8.46
2628 SOD2 6_103 0.000 118.12 0.0e+00 -7.20
5796 WTAP 6_103 0.000 175.68 0.0e+00 7.02
2629 MRPL18 6_103 0.000 109.26 0.0e+00 -6.69
5793 DYNLT1 6_103 0.000 66.15 0.0e+00 -6.33
5240 NLRC5 16_31 0.059 17.83 3.8e-06 6.20
7349 SYTL3 6_103 0.000 58.48 0.0e+00 5.73
1120 CETP 16_31 0.065 16.09 3.8e-06 5.71
7656 CATSPER2 15_16 0.629 20.97 4.8e-05 -4.75
4286 PRMT7 16_36 0.619 19.88 4.5e-05 4.56
12008 HPR 16_38 0.606 20.12 4.5e-05 -4.26
8865 FUT2 19_33 0.582 18.95 4.0e-05 -4.13
5795 PNLDC1 6_103 0.000 79.01 0.0e+00 4.09
4435 PSRC1 1_67 0.450 18.16 3.0e-05 -4.01Locus plots for genes and SNPs
ctwas_gene_res_sortz <- ctwas_gene_res[order(-abs(ctwas_gene_res$z)),]
n_plots <- 5
for (region_tag_plot in head(unique(ctwas_gene_res_sortz$region_tag), n_plots)){
ctwas_res_region <- ctwas_res[ctwas_res$region_tag==region_tag_plot,]
start <- min(ctwas_res_region$pos)
end <- max(ctwas_res_region$pos)
ctwas_res_region <- ctwas_res_region[order(ctwas_res_region$pos),]
ctwas_res_region_gene <- ctwas_res_region[ctwas_res_region$type=="gene",]
ctwas_res_region_snp <- ctwas_res_region[ctwas_res_region$type=="SNP",]
#region name
print(paste0("Region: ", region_tag_plot))
#table of genes in region
print(ctwas_res_region_gene[,report_cols])
par(mfrow=c(4,1))
#gene z scores
plot(ctwas_res_region_gene$pos, abs(ctwas_res_region_gene$z), xlab="Position", ylab="abs(gene_z)", xlim=c(start,end),
ylim=c(0,max(sig_thresh, abs(ctwas_res_region_gene$z))),
main=paste0("Region: ", region_tag_plot))
abline(h=sig_thresh,col="red",lty=2)
#significance threshold for SNPs
alpha_snp <- 5*10^(-8)
sig_thresh_snp <- qnorm(1-alpha_snp/2, lower=T)
#snp z scores
plot(ctwas_res_region_snp$pos, abs(ctwas_res_region_snp$z), xlab="Position", ylab="abs(snp_z)",xlim=c(start,end),
ylim=c(0,max(sig_thresh_snp, max(abs(ctwas_res_region_snp$z)))))
abline(h=sig_thresh_snp,col="purple",lty=2)
#gene pips
plot(ctwas_res_region_gene$pos, ctwas_res_region_gene$susie_pip, xlab="Position", ylab="Gene PIP", xlim=c(start,end), ylim=c(0,1))
abline(h=0.8,col="blue",lty=2)
#snp pips
plot(ctwas_res_region_snp$pos, ctwas_res_region_snp$susie_pip, xlab="Position", ylab="SNP PIP", xlim=c(start,end), ylim=c(0,1))
abline(h=0.8,col="blue",lty=2)
}[1] "Region: 6_104"
genename region_tag susie_pip mu2 PVE z
5799 SLC22A3 6_104 0 2321.99 0 -80.70
10435 LPA 6_104 0 3100.25 0 60.08
11043 RP1-81D8.3 6_104 0 165.64 0 -62.47
3449 PLG 6_104 0 437.35 0 48.04
1074 MAP3K4 6_104 0 148.80 0 24.06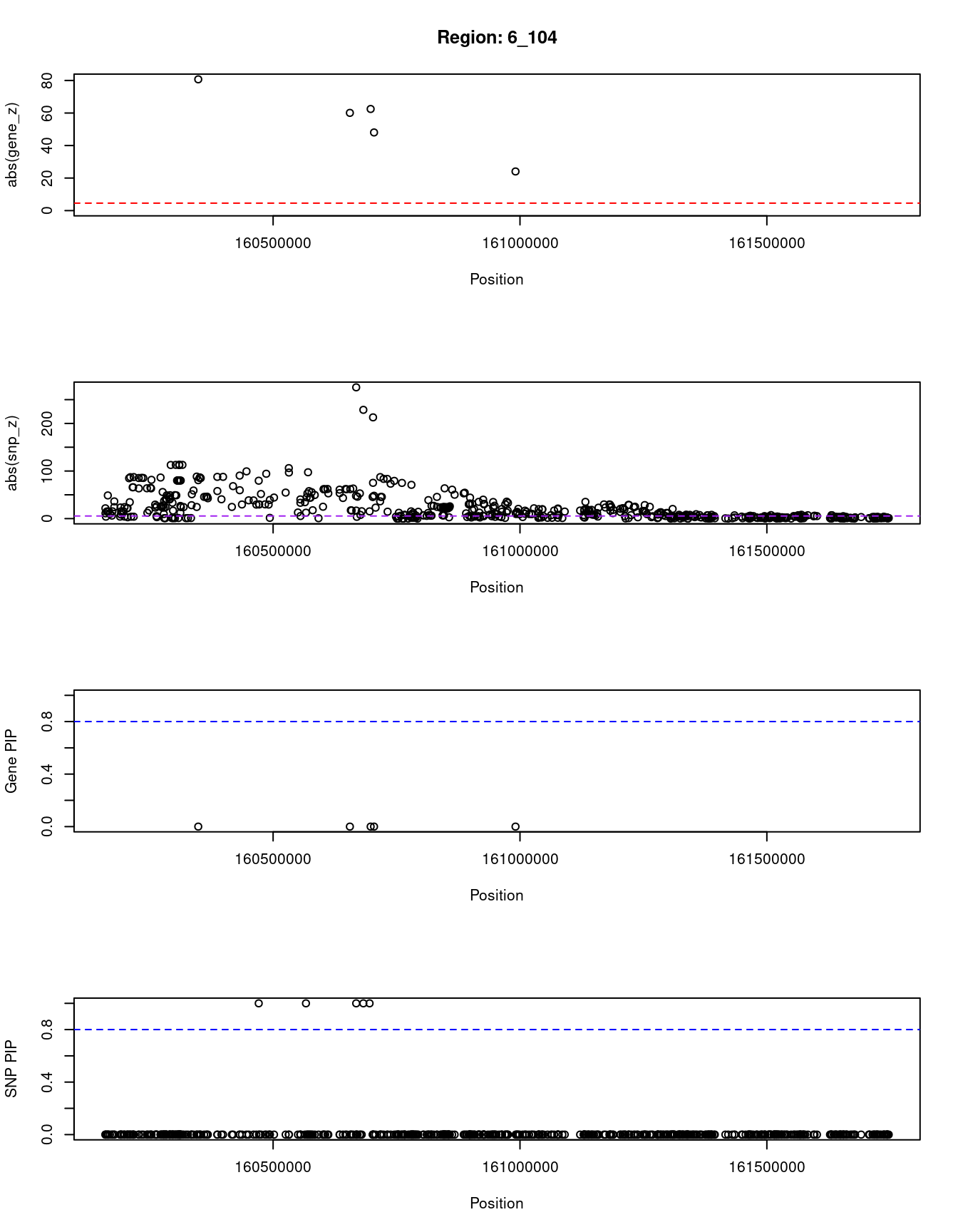
| Version | Author | Date |
|---|---|---|
| dfd2b5f | wesleycrouse | 2021-09-07 |
[1] "Region: 6_103"
genename region_tag susie_pip mu2 PVE z
911 SYNJ2 6_103 0 3.52 0 0.39
3456 SERAC1 6_103 0 17.11 0 2.74
12288 GTF2H5 6_103 0 6.42 0 -1.06
4067 TULP4 6_103 0 4.94 0 0.75
5793 DYNLT1 6_103 0 66.15 0 -6.33
7349 SYTL3 6_103 0 58.48 0 5.73
1290 EZR 6_103 0 8.33 0 -1.78
10532 C6orf99 6_103 0 3.29 0 -0.17
4070 RSPH3 6_103 0 26.92 0 3.37
7351 TAGAP 6_103 0 15.98 0 2.85
11375 RP3-393E18.2 6_103 0 135.84 0 -13.28
5796 WTAP 6_103 0 175.68 0 7.02
2628 SOD2 6_103 0 118.12 0 -7.20
3332 TCP1 6_103 0 16.71 0 -2.16
2629 MRPL18 6_103 0 109.26 0 -6.69
5795 PNLDC1 6_103 0 79.01 0 4.09
10185 IGF2R 6_103 0 20.68 0 -8.46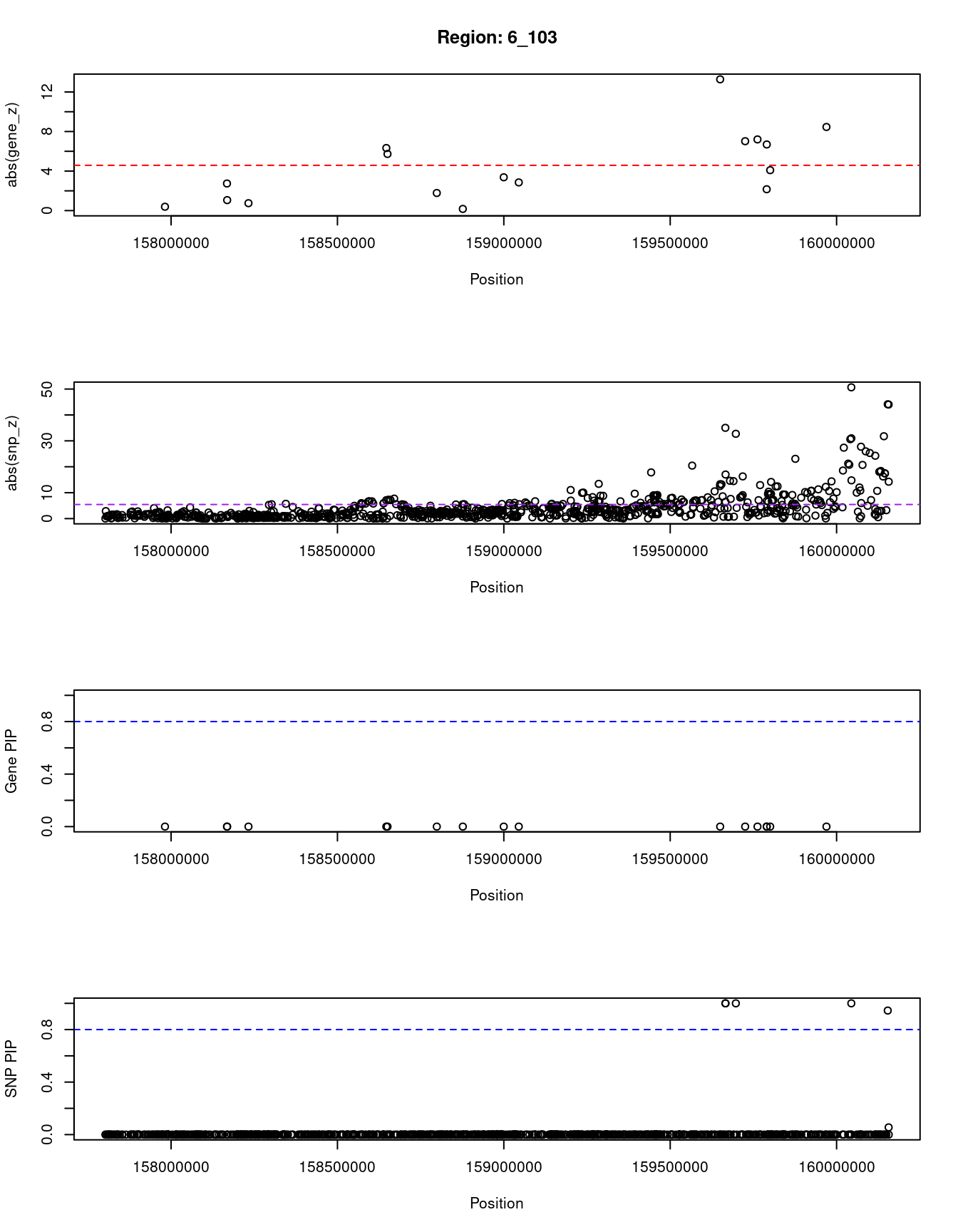
| Version | Author | Date |
|---|---|---|
| dfd2b5f | wesleycrouse | 2021-09-07 |
[1] "Region: 16_31"
genename region_tag susie_pip mu2 PVE z
6688 CES5A 16_31 0.086 5.29 1.7e-06 0.83
1124 GNAO1 16_31 0.060 3.24 7.1e-07 0.32
11561 RP11-461O7.1 16_31 0.078 4.73 1.3e-06 0.77
6695 AMFR 16_31 0.059 3.27 7.1e-07 0.65
7710 NUDT21 16_31 0.067 3.88 9.5e-07 0.61
3681 BBS2 16_31 0.062 3.51 7.9e-07 0.70
1122 MT3 16_31 0.060 3.22 7.1e-07 0.04
8094 MT1E 16_31 0.170 9.80 6.1e-06 -2.03
10727 MT1M 16_31 0.064 4.12 9.6e-07 1.29
10725 MT1A 16_31 0.110 7.56 3.0e-06 1.99
10386 MT1F 16_31 0.061 3.50 7.8e-07 0.84
9805 MT1X 16_31 0.066 3.75 9.1e-07 0.39
1740 NUP93 16_31 0.069 4.13 1.0e-06 -0.73
438 HERPUD1 16_31 0.065 4.12 9.7e-07 1.13
1120 CETP 16_31 0.065 16.09 3.8e-06 5.71
5240 NLRC5 16_31 0.059 17.83 3.8e-06 6.20
5239 CPNE2 16_31 0.064 3.61 8.4e-07 0.50
8472 FAM192A 16_31 0.072 4.48 1.2e-06 -1.03
6698 RSPRY1 16_31 0.100 6.02 2.2e-06 0.84
1745 PLLP 16_31 0.060 3.34 7.3e-07 -0.62
81 CX3CL1 16_31 0.064 3.73 8.7e-07 -0.84
1747 CCL17 16_31 0.082 5.36 1.6e-06 -1.29
52 CIAPIN1 16_31 0.065 3.87 9.2e-07 -0.83
1154 COQ9 16_31 0.078 4.90 1.4e-06 -1.01
3685 DOK4 16_31 0.059 3.13 6.8e-07 0.00
4628 CCDC102A 16_31 0.118 7.64 3.3e-06 1.69
10722 ADGRG1 16_31 0.059 3.10 6.6e-07 0.04
9366 ADGRG3 16_31 0.080 5.04 1.5e-06 1.05
5241 KATNB1 16_31 0.060 3.23 7.1e-07 -0.28
5242 KIFC3 16_31 0.068 3.98 9.8e-07 0.69
1754 USB1 16_31 0.065 3.77 9.0e-07 0.61
7571 ZNF319 16_31 0.061 3.35 7.5e-07 0.38
1753 MMP15 16_31 0.063 3.53 8.1e-07 0.47
729 CFAP20 16_31 0.081 5.14 1.5e-06 1.08
730 CSNK2A2 16_31 0.059 3.11 6.7e-07 0.10
9278 GINS3 16_31 0.073 4.46 1.2e-06 0.88
1757 NDRG4 16_31 0.064 3.66 8.6e-07 -0.56
3680 CNOT1 16_31 0.072 4.35 1.1e-06 0.85
1759 SLC38A7 16_31 0.081 5.13 1.5e-06 1.09
3684 GOT2 16_31 0.086 5.54 1.7e-06 -1.19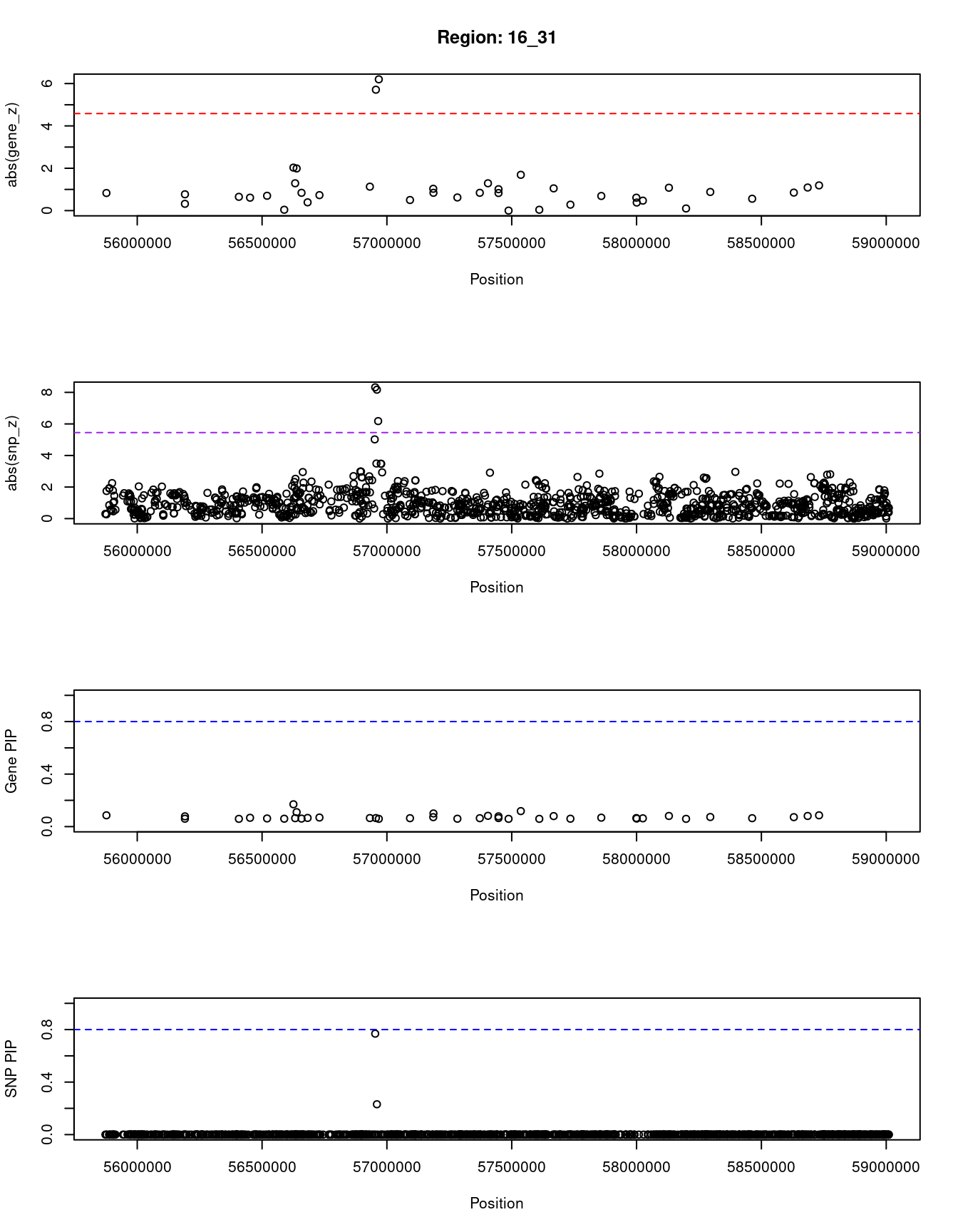
| Version | Author | Date |
|---|---|---|
| dfd2b5f | wesleycrouse | 2021-09-07 |
[1] "Region: 15_16"
genename region_tag susie_pip mu2 PVE z
1853 ZNF106 15_16 0.055 3.79 7.6e-07 1.00
9202 LRRC57 15_16 0.053 3.55 6.9e-07 0.63
6691 STARD9 15_16 0.054 3.69 7.3e-07 -0.66
5189 CDAN1 15_16 0.056 3.97 8.2e-07 0.66
3962 TTBK2 15_16 0.131 9.38 4.5e-06 -1.99
4903 TMEM62 15_16 0.056 3.89 7.9e-07 -0.83
7984 ADAL 15_16 0.052 3.48 6.6e-07 1.41
7985 LCMT2 15_16 0.133 9.49 4.6e-06 3.20
4898 TUBGCP4 15_16 0.057 4.03 8.4e-07 -1.71
5180 ZSCAN29 15_16 0.053 3.57 6.9e-07 -0.13
7702 MAP1A 15_16 0.198 12.12 8.7e-06 -3.40
7656 CATSPER2 15_16 0.629 20.97 4.8e-05 -4.75
7709 PDIA3 15_16 0.050 3.26 6.0e-07 -0.21
5178 MFAP1 15_16 0.117 8.64 3.7e-06 -2.80
1286 WDR76 15_16 0.126 9.11 4.2e-06 -2.83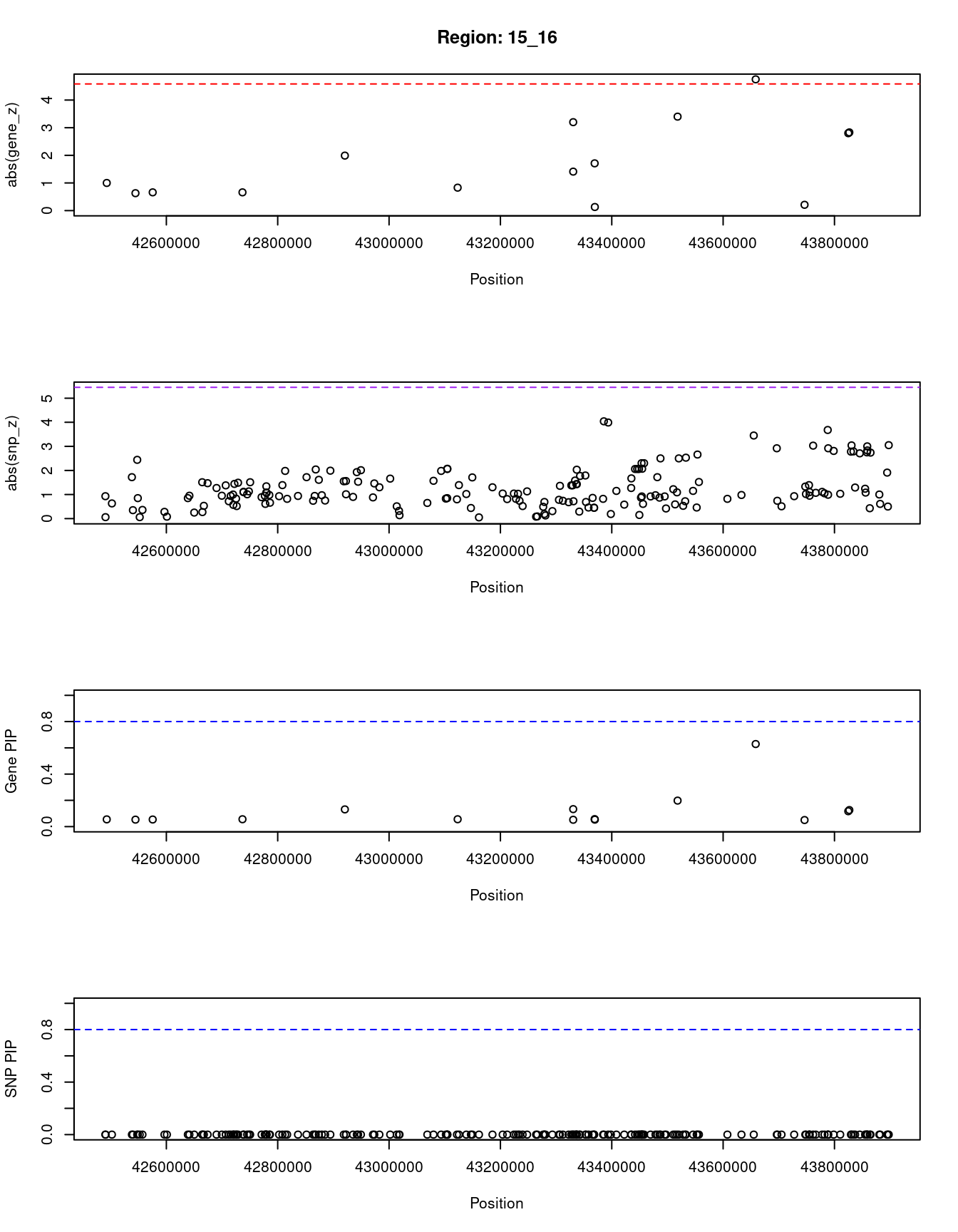
| Version | Author | Date |
|---|---|---|
| dfd2b5f | wesleycrouse | 2021-09-07 |
[1] "Region: 16_36"
genename region_tag susie_pip mu2 PVE z
11566 LINC00920 16_36 0.110 7.32 2.9e-06 1.42
7635 BEAN1 16_36 0.064 3.83 8.9e-07 0.51
7636 TK2 16_36 0.085 5.69 1.8e-06 -1.21
10968 CKLF 16_36 0.085 5.69 1.8e-06 1.21
1195 CMTM1 16_36 0.057 3.12 6.5e-07 0.04
5245 CMTM3 16_36 0.067 4.11 1.0e-06 -0.55
9452 CMTM4 16_36 0.109 7.27 2.9e-06 1.84
4626 DYNC1LI2 16_36 0.065 3.94 9.3e-07 -1.11
6700 NAE1 16_36 0.058 3.19 6.7e-07 -0.36
7643 FAM96B 16_36 0.060 3.47 7.7e-07 -0.30
8480 CES3 16_36 0.057 3.11 6.5e-07 -0.47
661 CBFB 16_36 0.060 3.40 7.4e-07 -0.73
3683 C16orf70 16_36 0.070 4.43 1.1e-06 0.63
10022 KIAA0895L 16_36 0.086 5.73 1.8e-06 -1.40
9065 EXOC3L1 16_36 0.057 3.11 6.5e-07 0.30
10715 E2F4 16_36 0.057 3.12 6.5e-07 -0.24
1737 ELMO3 16_36 0.151 9.38 5.2e-06 2.45
4629 SLC9A5 16_36 0.060 3.42 7.5e-07 0.77
4627 FHOD1 16_36 0.064 3.81 8.8e-07 0.88
6708 TPPP3 16_36 0.078 5.11 1.5e-06 -1.50
1748 CTCF 16_36 0.061 3.56 7.9e-07 -0.41
1749 ACD 16_36 0.058 3.19 6.7e-07 -0.15
1751 PARD6A 16_36 0.058 3.19 6.7e-07 -0.15
3584 ENKD1 16_36 0.057 3.13 6.5e-07 -0.06
5272 GFOD2 16_36 0.061 3.53 7.8e-07 -0.46
1741 TSNAXIP1 16_36 0.061 3.51 7.8e-07 1.08
5271 RANBP10 16_36 0.057 3.10 6.5e-07 -0.02
1739 NUTF2 16_36 0.057 3.10 6.4e-07 -0.04
6713 PSKH1 16_36 0.138 8.81 4.4e-06 -2.69
10714 PSMB10 16_36 0.057 3.10 6.4e-07 -0.13
9360 DDX28 16_36 0.165 9.98 6.0e-06 3.07
770 NFATC3 16_36 0.124 8.08 3.6e-06 -2.71
1765 ESRP2 16_36 0.098 6.60 2.4e-06 2.12
1764 PLA2G15 16_36 0.059 3.30 7.1e-07 -0.74
1763 SLC7A6 16_36 0.080 5.26 1.5e-06 -1.39
4286 PRMT7 16_36 0.619 19.88 4.5e-05 4.56
9563 ZFP90 16_36 0.096 6.47 2.3e-06 1.33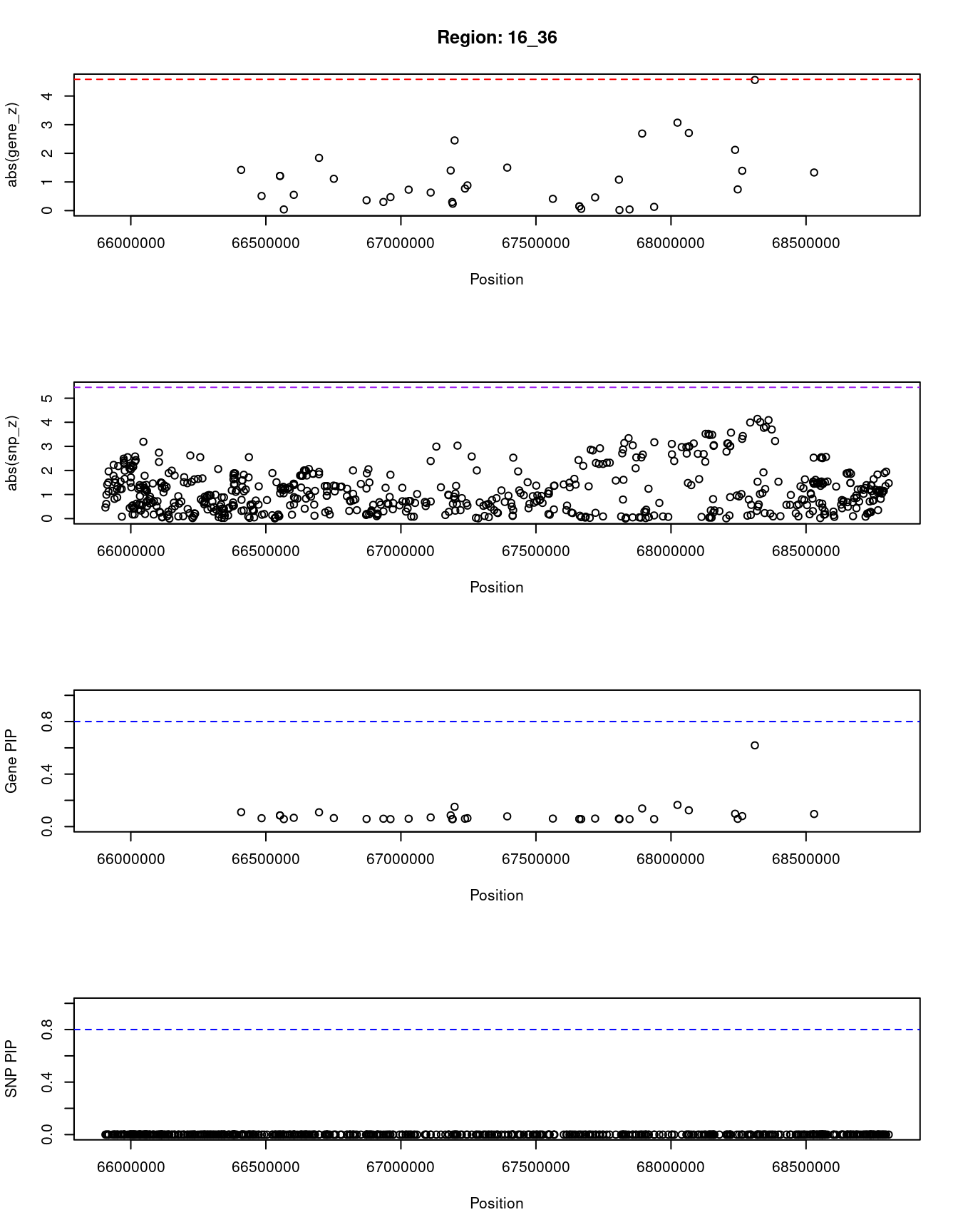
| Version | Author | Date |
|---|---|---|
| dfd2b5f | wesleycrouse | 2021-09-07 |
SNPs with highest PIPs
#snps with PIP>0.8 or 20 highest PIPs
head(ctwas_snp_res[order(-ctwas_snp_res$susie_pip),report_cols_snps],
max(sum(ctwas_snp_res$susie_pip>0.8), 20)) id region_tag susie_pip mu2 PVE z
376634 rs185885155 6_103 1.000 1298.05 4.7e-03 35.03
376636 rs2842983 6_103 1.000 461.56 1.7e-03 17.01
376644 rs117055069 6_103 1.000 624.86 2.3e-03 32.73
376751 rs688359 6_103 1.000 1814.23 6.6e-03 -50.66
376910 rs182443492 6_104 1.000 6801.55 2.5e-02 79.64
376928 rs141383002 6_104 1.000 8049.15 2.9e-02 -12.40
376955 rs56393506 6_104 1.000 67286.67 2.5e-01 275.91
376962 rs374071816 6_104 1.000 29248.69 1.1e-01 228.59
376963 rs9458002 6_104 1.000 6913.57 2.5e-02 15.22
821102 rs113345881 19_32 1.000 85.02 3.1e-04 -9.42
821105 rs12721109 19_32 1.000 67.73 2.5e-04 -7.62
775778 rs1801689 17_38 0.977 48.96 1.7e-04 6.65
376784 rs1443844 6_103 0.945 1461.62 5.0e-03 -44.12
821067 rs111794050 19_32 0.883 50.33 1.6e-04 -7.44
745890 rs12446515 16_31 0.769 73.96 2.1e-04 -8.32
821095 rs34878901 19_32 0.360 96.37 1.3e-04 7.49
745892 rs821840 16_31 0.231 71.57 6.0e-05 -8.17
821092 rs8106922 19_32 0.084 86.21 2.7e-05 7.20
376785 rs3818678 6_103 0.055 1462.14 3.0e-04 -44.02
377558 rs76667746 6_105 0.055 139.97 2.8e-05 5.50SNPs with largest effect sizes
#plot PIP vs effect size
plot(ctwas_snp_res$susie_pip, ctwas_snp_res$mu2, xlab="PIP", ylab="mu^2", main="SNP PIPs vs Effect Size")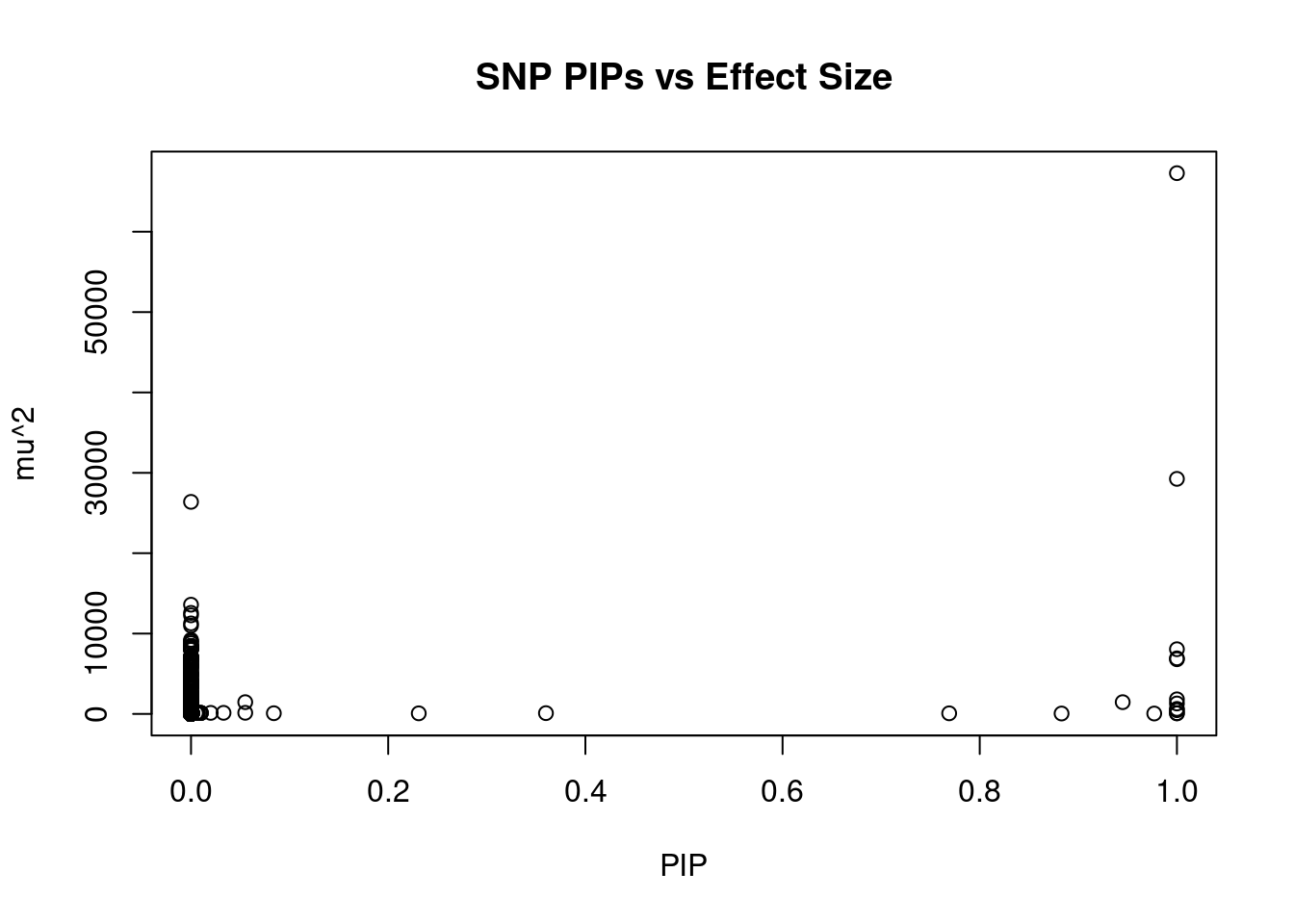
| Version | Author | Date |
|---|---|---|
| dfd2b5f | wesleycrouse | 2021-09-07 |
#SNPs with 50 largest effect sizes
head(ctwas_snp_res[order(-ctwas_snp_res$mu2),report_cols_snps],50) id region_tag susie_pip mu2 PVE z
376955 rs56393506 6_104 1 67286.67 2.5e-01 275.91
376962 rs374071816 6_104 1 29248.69 1.1e-01 228.59
376967 rs4252185 6_104 0 26385.89 0.0e+00 212.79
376921 rs3124784 6_104 0 13588.94 0.0e+00 106.12
376922 rs3127596 6_104 0 12573.24 0.0e+00 96.83
376915 rs3127599 6_104 0 12266.73 0.0e+00 94.24
376953 rs9365200 6_104 0 11262.91 0.0e+00 63.20
376919 rs117733303 6_104 0 10983.45 0.0e+00 44.24
376899 rs1510226 6_104 0 9214.94 0.0e+00 40.59
376971 rs783147 6_104 0 9034.42 0.0e+00 -87.24
376906 rs9355288 6_104 0 9012.73 0.0e+00 99.30
376966 rs2314851 6_104 0 8795.09 0.0e+00 75.18
376879 rs3106167 6_104 0 8450.88 0.0e+00 112.93
376870 rs3106169 6_104 0 8450.04 0.0e+00 112.93
376871 rs3127598 6_104 0 8447.02 0.0e+00 112.91
376863 rs11755965 6_104 0 8443.74 0.0e+00 112.90
376854 rs12194962 6_104 0 8422.52 0.0e+00 112.68
376872 rs3127597 6_104 0 8374.08 0.0e+00 112.65
376943 rs9355814 6_104 0 8250.71 0.0e+00 -62.25
376948 rs36115561 6_104 0 8246.29 0.0e+00 -62.12
376950 rs2092931 6_104 0 8225.50 0.0e+00 61.87
376940 rs6455692 6_104 0 8224.87 0.0e+00 -61.80
376941 rs7769720 6_104 0 8219.46 0.0e+00 -61.75
376942 rs6932014 6_104 0 8218.89 0.0e+00 -61.75
376949 rs10945683 6_104 0 8210.24 0.0e+00 -61.76
376974 rs9458016 6_104 0 8079.69 0.0e+00 -83.48
376928 rs141383002 6_104 1 8049.15 2.9e-02 -12.40
376945 rs7765684 6_104 0 8045.24 0.0e+00 -60.80
376975 rs4252125 6_104 0 8035.29 0.0e+00 -83.37
376959 rs1590183 6_104 0 7973.39 0.0e+00 52.93
376978 rs80276688 6_104 0 7153.35 0.0e+00 -79.28
376936 rs117945468 6_104 0 7145.95 0.0e+00 17.54
376946 rs7746273 6_104 0 7141.24 0.0e+00 -53.48
376970 rs14224 6_104 0 7095.04 0.0e+00 36.31
376956 rs117830977 6_104 0 7039.47 0.0e+00 16.81
376900 rs2872317 6_104 0 6988.96 0.0e+00 87.72
376891 rs624319 6_104 0 6984.47 0.0e+00 -86.58
376897 rs2313453 6_104 0 6981.38 0.0e+00 87.68
376963 rs9458002 6_104 1 6913.57 2.5e-02 15.22
376910 rs182443492 6_104 1 6801.55 2.5e-02 79.64
376961 rs58247541 6_104 0 6795.61 2.3e-06 15.50
376933 rs9457943 6_104 0 6720.67 0.0e+00 -43.87
376934 rs6923877 6_104 0 6718.91 0.0e+00 -43.96
376890 rs637614 6_104 0 6714.07 0.0e+00 -85.42
376929 rs9365174 6_104 0 6683.38 0.0e+00 -44.67
376892 rs486339 6_104 0 6682.45 0.0e+00 -85.10
376965 rs1950562 6_104 0 6535.18 0.0e+00 45.64
376964 rs1864450 6_104 0 6534.46 0.0e+00 45.69
376903 rs388170 6_104 0 6413.35 0.0e+00 90.40
376987 rs60198722 6_104 0 6180.19 0.0e+00 -74.97SNPs with highest PVE
#SNPs with 50 highest pve
head(ctwas_snp_res[order(-ctwas_snp_res$PVE),report_cols_snps],50) id region_tag susie_pip mu2 PVE z
376955 rs56393506 6_104 1.000 67286.67 2.5e-01 275.91
376962 rs374071816 6_104 1.000 29248.69 1.1e-01 228.59
376928 rs141383002 6_104 1.000 8049.15 2.9e-02 -12.40
376910 rs182443492 6_104 1.000 6801.55 2.5e-02 79.64
376963 rs9458002 6_104 1.000 6913.57 2.5e-02 15.22
376751 rs688359 6_103 1.000 1814.23 6.6e-03 -50.66
376784 rs1443844 6_103 0.945 1461.62 5.0e-03 -44.12
376634 rs185885155 6_103 1.000 1298.05 4.7e-03 35.03
376644 rs117055069 6_103 1.000 624.86 2.3e-03 32.73
376636 rs2842983 6_103 1.000 461.56 1.7e-03 17.01
821102 rs113345881 19_32 1.000 85.02 3.1e-04 -9.42
376785 rs3818678 6_103 0.055 1462.14 3.0e-04 -44.02
821105 rs12721109 19_32 1.000 67.73 2.5e-04 -7.62
745890 rs12446515 16_31 0.769 73.96 2.1e-04 -8.32
775778 rs1801689 17_38 0.977 48.96 1.7e-04 6.65
821067 rs111794050 19_32 0.883 50.33 1.6e-04 -7.44
821095 rs34878901 19_32 0.360 96.37 1.3e-04 7.49
745892 rs821840 16_31 0.231 71.57 6.0e-05 -8.17
377558 rs76667746 6_105 0.055 139.97 2.8e-05 5.50
821092 rs8106922 19_32 0.084 86.21 2.7e-05 7.20
377931 rs55902653 6_106 0.033 134.66 1.6e-05 -5.21
377560 rs2023073 6_105 0.020 129.90 9.7e-06 5.30
810578 rs147985405 19_9 0.010 121.53 4.6e-06 -5.14
810571 rs138175288 19_9 0.010 121.38 4.5e-06 -5.14
810572 rs138294113 19_9 0.010 120.96 4.3e-06 -5.13
810575 rs112552009 19_9 0.009 119.64 3.8e-06 -5.10
810573 rs73015020 19_9 0.007 117.73 3.1e-06 -5.06
810570 rs55997232 19_9 0.007 117.26 2.9e-06 -5.05
810576 rs10412048 19_9 0.006 115.37 2.4e-06 -5.01
376961 rs58247541 6_104 0.000 6795.61 2.3e-06 15.50
810574 rs77140532 19_9 0.005 113.47 1.9e-06 -4.97
810580 rs2228671 19_9 0.001 101.97 5.5e-07 -4.67
680870 rs138414965 14_4 0.001 102.77 5.3e-07 -4.43
810579 rs17248769 19_9 0.001 100.95 4.9e-07 -4.64
380295 rs9346600 6_111 0.001 99.98 4.0e-07 -4.36
572836 rs11606904 11_21 0.001 98.59 3.4e-07 -4.33
187068 rs79694228 3_95 0.001 96.73 2.7e-07 4.31
377492 rs142568872 6_105 0.001 96.49 2.6e-07 4.31
810569 rs9305020 19_9 0.001 93.94 2.3e-07 -4.45
375772 rs2886418 6_102 0.001 95.39 2.2e-07 4.25
808188 rs146795208 19_5 0.001 93.93 2.2e-07 4.23
142257 rs17043569 3_4 0.001 94.56 2.0e-07 -4.23
377548 rs78513344 6_105 0.001 93.40 1.8e-07 -4.23
15752 rs12061007 1_34 0.001 92.34 1.7e-07 -4.18
122251 rs11887867 2_111 0.000 92.34 1.7e-07 4.19
675328 rs10508138 13_54 0.000 92.41 1.7e-07 4.18
406030 rs12535060 7_46 0.000 92.01 1.6e-07 4.17
377403 rs185899693 6_105 0.000 91.59 1.5e-07 -4.17
425479 rs72611550 7_85 0.000 91.44 1.5e-07 4.18
821097 rs440446 19_32 0.000 119.52 1.5e-07 -5.86SNPs with largest z scores
#SNPs with 50 largest z scores
head(ctwas_snp_res[order(-abs(ctwas_snp_res$z)),report_cols_snps],50) id region_tag susie_pip mu2 PVE z
376955 rs56393506 6_104 1 67286.67 0.250 275.91
376962 rs374071816 6_104 1 29248.69 0.110 228.59
376967 rs4252185 6_104 0 26385.89 0.000 212.79
376870 rs3106169 6_104 0 8450.04 0.000 112.93
376879 rs3106167 6_104 0 8450.88 0.000 112.93
376871 rs3127598 6_104 0 8447.02 0.000 112.91
376863 rs11755965 6_104 0 8443.74 0.000 112.90
376854 rs12194962 6_104 0 8422.52 0.000 112.68
376872 rs3127597 6_104 0 8374.08 0.000 112.65
376921 rs3124784 6_104 0 13588.94 0.000 106.12
376906 rs9355288 6_104 0 9012.73 0.000 99.30
376931 rs6903649 6_104 0 5988.78 0.000 97.39
376922 rs3127596 6_104 0 12573.24 0.000 96.83
376915 rs3127599 6_104 0 12266.73 0.000 94.24
376903 rs388170 6_104 0 6413.35 0.000 90.40
376888 rs146184004 6_104 0 5698.91 0.000 88.29
376900 rs2872317 6_104 0 6988.96 0.000 87.72
376897 rs2313453 6_104 0 6981.38 0.000 87.68
376816 rs3103352 6_104 0 6063.78 0.000 87.29
376971 rs783147 6_104 0 9034.42 0.000 -87.24
376812 rs3101821 6_104 0 6028.82 0.000 87.08
376891 rs624319 6_104 0 6984.47 0.000 -86.58
376833 rs3119311 6_104 0 5196.07 0.000 86.32
376820 rs3119308 6_104 0 5929.80 0.000 85.71
376890 rs637614 6_104 0 6714.07 0.000 -85.42
376821 rs10945658 6_104 0 5830.82 0.000 85.40
376818 rs12205178 6_104 0 5842.91 0.000 85.33
376892 rs486339 6_104 0 6682.45 0.000 -85.10
376810 rs148015788 6_104 0 5852.90 0.000 84.84
376974 rs9458016 6_104 0 8079.69 0.000 -83.48
376975 rs4252125 6_104 0 8035.29 0.000 -83.37
376827 rs3127579 6_104 0 5394.47 0.000 81.48
376889 rs555754 6_104 0 6057.12 0.000 -80.70
376868 rs452867 6_104 0 4915.40 0.000 -80.08
376877 rs367334 6_104 0 4922.25 0.000 -80.03
376865 rs589931 6_104 0 4922.56 0.000 -80.02
376866 rs600584 6_104 0 4922.53 0.000 -80.02
376874 rs60425481 6_104 0 5460.48 0.000 -80.02
376867 rs434953 6_104 0 4920.93 0.000 -80.01
376873 rs380498 6_104 0 4919.48 0.000 -80.00
376910 rs182443492 6_104 1 6801.55 0.025 79.64
376978 rs80276688 6_104 0 7153.35 0.000 -79.28
376966 rs2314851 6_104 0 8795.09 0.000 75.18
376987 rs60198722 6_104 0 6180.19 0.000 -74.97
376977 rs4252150 6_104 0 4591.96 0.000 -73.36
376998 rs1620875 6_104 0 1256.16 0.000 -70.93
376902 rs79648238 6_104 0 2850.81 0.000 68.20
376815 rs595374 6_104 0 4039.81 0.000 -65.88
376814 rs610206 6_104 0 4023.60 0.000 -65.75
376825 rs316013 6_104 0 3729.57 0.000 -64.84Gene set enrichment for genes with PIP>0.8
#GO enrichment analysis
library(enrichR)Welcome to enrichR
Checking connection ... Enrichr ... Connection is Live!
FlyEnrichr ... Connection is available!
WormEnrichr ... Connection is available!
YeastEnrichr ... Connection is available!
FishEnrichr ... Connection is available!dbs <- c("GO_Biological_Process_2021", "GO_Cellular_Component_2021", "GO_Molecular_Function_2021")
genes <- ctwas_gene_res$genename[ctwas_gene_res$susie_pip>0.8]
#number of genes for gene set enrichment
length(genes)[1] 0if (length(genes)>0){
GO_enrichment <- enrichr(genes, dbs)
for (db in dbs){
print(db)
df <- GO_enrichment[[db]]
df <- df[df$Adjusted.P.value<0.05,c("Term", "Overlap", "Adjusted.P.value", "Genes")]
print(df)
}
#DisGeNET enrichment
# devtools::install_bitbucket("ibi_group/disgenet2r")
library(disgenet2r)
disgenet_api_key <- get_disgenet_api_key(
email = "wesleycrouse@gmail.com",
password = "uchicago1" )
Sys.setenv(DISGENET_API_KEY= disgenet_api_key)
res_enrich <-disease_enrichment(entities=genes, vocabulary = "HGNC",
database = "CURATED" )
df <- res_enrich@qresult[1:10, c("Description", "FDR", "Ratio", "BgRatio")]
print(df)
#WebGestalt enrichment
library(WebGestaltR)
background <- ctwas_gene_res$genename
#listGeneSet()
databases <- c("pathway_KEGG", "disease_GLAD4U", "disease_OMIM")
enrichResult <- WebGestaltR(enrichMethod="ORA", organism="hsapiens",
interestGene=genes, referenceGene=background,
enrichDatabase=databases, interestGeneType="genesymbol",
referenceGeneType="genesymbol", isOutput=F)
print(enrichResult[,c("description", "size", "overlap", "FDR", "database", "userId")])
}
sessionInfo()R version 3.6.1 (2019-07-05)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: Scientific Linux 7.4 (Nitrogen)
Matrix products: default
BLAS/LAPACK: /software/openblas-0.2.19-el7-x86_64/lib/libopenblas_haswellp-r0.2.19.so
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
[3] LC_TIME=en_US.UTF-8 LC_COLLATE=en_US.UTF-8
[5] LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=en_US.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
attached base packages:
[1] stats graphics grDevices utils datasets methods base
other attached packages:
[1] enrichR_3.0 cowplot_1.0.0 ggplot2_3.3.3
loaded via a namespace (and not attached):
[1] Biobase_2.44.0 httr_1.4.1
[3] bit64_4.0.5 assertthat_0.2.1
[5] stats4_3.6.1 blob_1.2.1
[7] BSgenome_1.52.0 GenomeInfoDbData_1.2.1
[9] Rsamtools_2.0.0 yaml_2.2.0
[11] progress_1.2.2 pillar_1.6.1
[13] RSQLite_2.2.7 lattice_0.20-38
[15] glue_1.4.2 digest_0.6.20
[17] GenomicRanges_1.36.0 promises_1.0.1
[19] XVector_0.24.0 colorspace_1.4-1
[21] htmltools_0.3.6 httpuv_1.5.1
[23] Matrix_1.2-18 XML_3.98-1.20
[25] pkgconfig_2.0.3 biomaRt_2.40.1
[27] zlibbioc_1.30.0 purrr_0.3.4
[29] scales_1.1.0 whisker_0.3-2
[31] later_0.8.0 BiocParallel_1.18.0
[33] git2r_0.26.1 tibble_3.1.2
[35] farver_2.1.0 generics_0.0.2
[37] IRanges_2.18.1 ellipsis_0.3.2
[39] withr_2.4.1 cachem_1.0.5
[41] SummarizedExperiment_1.14.1 GenomicFeatures_1.36.3
[43] BiocGenerics_0.30.0 magrittr_2.0.1
[45] crayon_1.4.1 memoise_2.0.0
[47] evaluate_0.14 fs_1.3.1
[49] fansi_0.5.0 tools_3.6.1
[51] data.table_1.14.0 prettyunits_1.0.2
[53] hms_1.1.0 lifecycle_1.0.0
[55] matrixStats_0.57.0 stringr_1.4.0
[57] S4Vectors_0.22.1 munsell_0.5.0
[59] DelayedArray_0.10.0 AnnotationDbi_1.46.0
[61] Biostrings_2.52.0 compiler_3.6.1
[63] GenomeInfoDb_1.20.0 rlang_0.4.11
[65] grid_3.6.1 RCurl_1.98-1.1
[67] rjson_0.2.20 VariantAnnotation_1.30.1
[69] labeling_0.3 bitops_1.0-6
[71] rmarkdown_1.13 gtable_0.3.0
[73] curl_3.3 DBI_1.1.1
[75] R6_2.5.0 GenomicAlignments_1.20.1
[77] dplyr_1.0.7 knitr_1.23
[79] rtracklayer_1.44.0 utf8_1.2.1
[81] fastmap_1.1.0 bit_4.0.4
[83] workflowr_1.6.2 rprojroot_2.0.2
[85] stringi_1.4.3 parallel_3.6.1
[87] Rcpp_1.0.6 vctrs_0.3.8
[89] tidyselect_1.1.0 xfun_0.8