Diabetes diagnosed by doctor - Adipose_Subcutaneous
wesleycrouse
2021-09-7
Last updated: 2021-09-13
Checks: 7 0
Knit directory: ctwas_applied/
This reproducible R Markdown analysis was created with workflowr (version 1.6.2). The Checks tab describes the reproducibility checks that were applied when the results were created. The Past versions tab lists the development history.
Great! Since the R Markdown file has been committed to the Git repository, you know the exact version of the code that produced these results.
Great job! The global environment was empty. Objects defined in the global environment can affect the analysis in your R Markdown file in unknown ways. For reproduciblity it’s best to always run the code in an empty environment.
The command set.seed(20210726) was run prior to running the code in the R Markdown file. Setting a seed ensures that any results that rely on randomness, e.g. subsampling or permutations, are reproducible.
Great job! Recording the operating system, R version, and package versions is critical for reproducibility.
Nice! There were no cached chunks for this analysis, so you can be confident that you successfully produced the results during this run.
Great job! Using relative paths to the files within your workflowr project makes it easier to run your code on other machines.
Great! You are using Git for version control. Tracking code development and connecting the code version to the results is critical for reproducibility.
The results in this page were generated with repository version 72c8ef7. See the Past versions tab to see a history of the changes made to the R Markdown and HTML files.
Note that you need to be careful to ensure that all relevant files for the analysis have been committed to Git prior to generating the results (you can use wflow_publish or wflow_git_commit). workflowr only checks the R Markdown file, but you know if there are other scripts or data files that it depends on. Below is the status of the Git repository when the results were generated:
working directory clean
Note that any generated files, e.g. HTML, png, CSS, etc., are not included in this status report because it is ok for generated content to have uncommitted changes.
These are the previous versions of the repository in which changes were made to the R Markdown (analysis/ukb-a-306_Adipose_Subcutaneous_known.Rmd) and HTML (docs/ukb-a-306_Adipose_Subcutaneous_known.html) files. If you’ve configured a remote Git repository (see ?wflow_git_remote), click on the hyperlinks in the table below to view the files as they were in that past version.
| File | Version | Author | Date | Message |
|---|---|---|---|---|
| Rmd | 72c8ef7 | wesleycrouse | 2021-09-13 | changing mart for biomart |
| Rmd | a49c40e | wesleycrouse | 2021-09-13 | updating with bystander analysis |
| html | 7e22565 | wesleycrouse | 2021-09-13 | updating reports |
| html | 3a7fbc1 | wesleycrouse | 2021-09-08 | generating reports for known annotations |
| Rmd | cbf7408 | wesleycrouse | 2021-09-08 | adding enrichment to reports |
Overview
These are the results of a ctwas analysis of the UK Biobank trait Diabetes diagnosed by doctor using Adipose_Subcutaneous gene weights.
The GWAS was conducted by the Neale Lab, and the biomarker traits we analyzed are discussed here. Summary statistics were obtained from IEU OpenGWAS using GWAS ID: ukb-a-306. Results were obtained from from IEU rather than Neale Lab because they are in a standardard format (GWAS VCF). Note that 3 of the 34 biomarker traits were not available from IEU and were excluded from analysis.
The weights are mashr GTEx v8 models on Adipose_Subcutaneous eQTL obtained from PredictDB. We performed a full harmonization of the variants, including recovering strand ambiguous variants. This procedure is discussed in a separate document. (TO-DO: add report that describes harmonization)
LD matrices were computed from a 10% subset of Neale lab subjects. Subjects were matched using the plate and well information from genotyping. We included only biallelic variants with MAF>0.01 in the original Neale Lab GWAS. (TO-DO: add more details [number of subjects, variants, etc])
Weight QC
TO-DO: add enhanced QC reporting (total number of weights, why each variant was missing for all genes)
qclist_all <- list()
qc_files <- paste0(results_dir, "/", list.files(results_dir, pattern="exprqc.Rd"))
for (i in 1:length(qc_files)){
load(qc_files[i])
chr <- unlist(strsplit(rev(unlist(strsplit(qc_files[i], "_")))[1], "[.]"))[1]
qclist_all[[chr]] <- cbind(do.call(rbind, lapply(qclist,unlist)), as.numeric(substring(chr,4)))
}
qclist_all <- data.frame(do.call(rbind, qclist_all))
colnames(qclist_all)[ncol(qclist_all)] <- "chr"
rm(qclist, wgtlist, z_gene_chr)
#number of imputed weights
nrow(qclist_all)[1] 12414#number of imputed weights by chromosome
table(qclist_all$chr)
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15
1223 885 712 493 574 708 607 468 472 481 750 671 248 411 431
16 17 18 19 20 21 22
580 758 186 940 370 138 308 #proportion of imputed weights without missing variants
mean(qclist_all$nmiss==0)[1] 0.7137909Load ctwas results
#load ctwas results
ctwas_res <- data.table::fread(paste0(results_dir, "/", analysis_id, "_ctwas.susieIrss.txt"))
#make unique identifier for regions
ctwas_res$region_tag <- paste(ctwas_res$region_tag1, ctwas_res$region_tag2, sep="_")
#load z scores for SNPs and collect sample size
load(paste0(results_dir, "/", analysis_id, "_expr_z_snp.Rd"))
sample_size <- z_snp$ss
sample_size <- as.numeric(names(which.max(table(sample_size))))
#compute PVE for each gene/SNP
ctwas_res$PVE = ctwas_res$susie_pip*ctwas_res$mu2/sample_size
#separate gene and SNP results
ctwas_gene_res <- ctwas_res[ctwas_res$type == "gene", ]
ctwas_gene_res <- data.frame(ctwas_gene_res)
ctwas_snp_res <- ctwas_res[ctwas_res$type == "SNP", ]
ctwas_snp_res <- data.frame(ctwas_snp_res)
#add gene information to results
sqlite <- RSQLite::dbDriver("SQLite")
db = RSQLite::dbConnect(sqlite, paste0("/project2/compbio/predictdb/mashr_models/mashr_", weight, ".db"))
query <- function(...) RSQLite::dbGetQuery(db, ...)
gene_info <- query("select gene, genename from extra")
gene_info <- query("select gene, genename, gene_type from extra")
RSQLite::dbDisconnect(db)
ctwas_gene_res <- cbind(ctwas_gene_res, gene_info[sapply(ctwas_gene_res$id, match, gene_info$gene), c("genename", "gene_type")])
#add z scores to results
load(paste0(results_dir, "/", analysis_id, "_expr_z_gene.Rd"))
ctwas_gene_res$z <- z_gene[ctwas_gene_res$id,]$z
z_snp <- z_snp[z_snp$id %in% ctwas_snp_res$id,]
ctwas_snp_res$z <- z_snp$z[match(ctwas_snp_res$id, z_snp$id)]
#formatting and rounding for tables
ctwas_gene_res$z <- round(ctwas_gene_res$z,2)
ctwas_snp_res$z <- round(ctwas_snp_res$z,2)
ctwas_gene_res$susie_pip <- round(ctwas_gene_res$susie_pip,3)
ctwas_snp_res$susie_pip <- round(ctwas_snp_res$susie_pip,3)
ctwas_gene_res$mu2 <- round(ctwas_gene_res$mu2,2)
ctwas_snp_res$mu2 <- round(ctwas_snp_res$mu2,2)
ctwas_gene_res$PVE <- signif(ctwas_gene_res$PVE, 2)
ctwas_snp_res$PVE <- signif(ctwas_snp_res$PVE, 2)
#merge gene and snp results with added information
ctwas_snp_res$genename=NA
ctwas_snp_res$gene_type=NA
ctwas_res <- rbind(ctwas_gene_res,
ctwas_snp_res[,colnames(ctwas_gene_res)])
#store columns to report
report_cols <- colnames(ctwas_gene_res)[!(colnames(ctwas_gene_res) %in% c("type", "region_tag1", "region_tag2", "cs_index", "gene_type", "z_flag", "id", "chrom", "pos"))]
first_cols <- c("genename", "region_tag")
report_cols <- c(first_cols, report_cols[!(report_cols %in% first_cols)])
report_cols_snps <- c("id", report_cols[-1])
#get number of SNPs from s1 results; adjust for thin
ctwas_res_s1 <- data.table::fread(paste0(results_dir, "/", analysis_id, "_ctwas.s1.susieIrss.txt"))
n_snps <- sum(ctwas_res_s1$type=="SNP")/thin
rm(ctwas_res_s1)Check convergence of parameters
library(ggplot2)
library(cowplot)
********************************************************Note: As of version 1.0.0, cowplot does not change the default ggplot2 theme anymore. To recover the previous behavior, execute:
theme_set(theme_cowplot())********************************************************load(paste0(results_dir, "/", analysis_id, "_ctwas.s2.susieIrssres.Rd"))
df <- data.frame(niter = rep(1:ncol(group_prior_rec), 2),
value = c(group_prior_rec[1,], group_prior_rec[2,]),
group = rep(c("Gene", "SNP"), each = ncol(group_prior_rec)))
df$group <- as.factor(df$group)
df$value[df$group=="SNP"] <- df$value[df$group=="SNP"]*thin #adjust parameter to account for thin argument
p_pi <- ggplot(df, aes(x=niter, y=value, group=group)) +
geom_line(aes(color=group)) +
geom_point(aes(color=group)) +
xlab("Iteration") + ylab(bquote(pi)) +
ggtitle("Prior mean") +
theme_cowplot()
df <- data.frame(niter = rep(1:ncol(group_prior_var_rec), 2),
value = c(group_prior_var_rec[1,], group_prior_var_rec[2,]),
group = rep(c("Gene", "SNP"), each = ncol(group_prior_var_rec)))
df$group <- as.factor(df$group)
p_sigma2 <- ggplot(df, aes(x=niter, y=value, group=group)) +
geom_line(aes(color=group)) +
geom_point(aes(color=group)) +
xlab("Iteration") + ylab(bquote(sigma^2)) +
ggtitle("Prior variance") +
theme_cowplot()
plot_grid(p_pi, p_sigma2)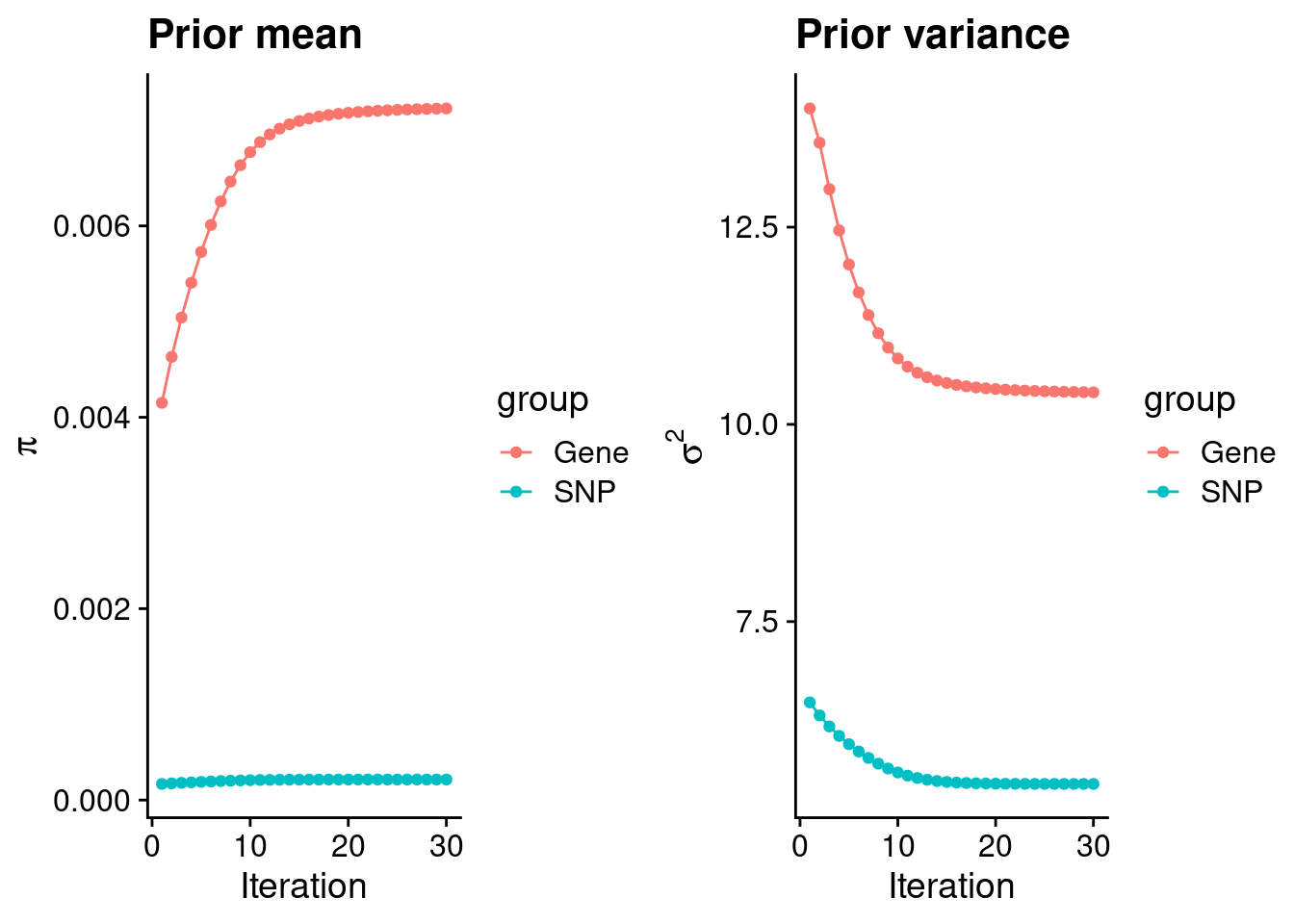
| Version | Author | Date |
|---|---|---|
| 3a7fbc1 | wesleycrouse | 2021-09-08 |
#estimated group prior
estimated_group_prior <- group_prior_rec[,ncol(group_prior_rec)]
names(estimated_group_prior) <- c("gene", "snp")
estimated_group_prior["snp"] <- estimated_group_prior["snp"]*thin #adjust parameter to account for thin argument
print(estimated_group_prior) gene snp
0.0072273144 0.0002145499 #estimated group prior variance
estimated_group_prior_var <- group_prior_var_rec[,ncol(group_prior_var_rec)]
names(estimated_group_prior_var) <- c("gene", "snp")
print(estimated_group_prior_var) gene snp
10.406176 5.441692 #report sample size
print(sample_size)[1] 336473#report group size
group_size <- c(nrow(ctwas_gene_res), n_snps)
print(group_size)[1] 12414 7535010#estimated group PVE
estimated_group_pve <- estimated_group_prior_var*estimated_group_prior*group_size/sample_size #check PVE calculation
names(estimated_group_pve) <- c("gene", "snp")
print(estimated_group_pve) gene snp
0.002774787 0.026145432 #compare sum(PIP*mu2/sample_size) with above PVE calculation
c(sum(ctwas_gene_res$PVE),sum(ctwas_snp_res$PVE))[1] 0.0150731 0.3780328Genes with highest PIPs
#distribution of PIPs
hist(ctwas_gene_res$susie_pip, xlim=c(0,1), main="Distribution of Gene PIPs")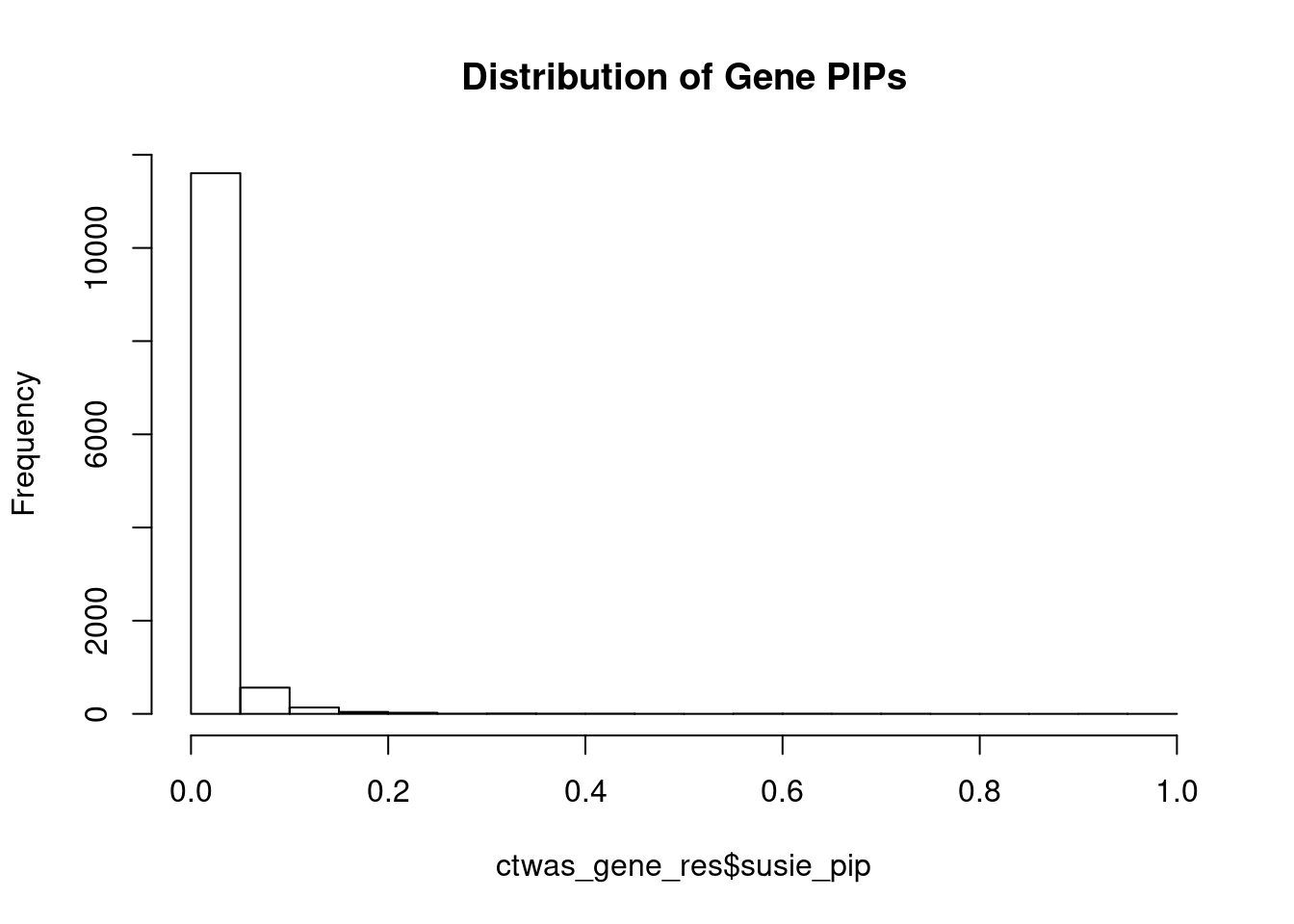
| Version | Author | Date |
|---|---|---|
| 3a7fbc1 | wesleycrouse | 2021-09-08 |
#genes with PIP>0.8 or 20 highest PIPs
head(ctwas_gene_res[order(-ctwas_gene_res$susie_pip),report_cols], max(sum(ctwas_gene_res$susie_pip>0.8), 20)) genename region_tag susie_pip mu2 PVE z
3633 CCND2 12_4 0.998 92.56 2.7e-04 9.86
13893 GTF2I 7_48 0.938 27.22 7.6e-05 -5.11
7923 INHBB 2_70 0.902 22.20 5.9e-05 -4.47
356 ANK1 8_36 0.829 40.62 1.0e-04 -6.50
7067 NUS1 6_78 0.745 22.23 4.9e-05 4.44
7048 JAZF1 7_23 0.728 53.35 1.2e-04 7.66
1866 SLCO4A1 20_37 0.704 23.37 4.9e-05 -4.30
7749 C16orf59 16_2 0.696 23.77 4.9e-05 -3.88
152 TFAP2B 6_38 0.657 28.53 5.6e-05 -5.10
7109 LONRF1 8_15 0.655 25.77 5.0e-05 4.08
3647 CCDC92 12_75 0.649 25.81 5.0e-05 4.43
13258 LINC01184 5_78 0.647 21.34 4.1e-05 -4.05
11487 ZNF34 8_94 0.639 25.48 4.8e-05 -4.72
3200 SNX4 3_78 0.611 22.47 4.1e-05 -3.83
9518 MANEA 6_65 0.600 26.82 4.8e-05 3.99
2723 WFS1 4_7 0.596 63.57 1.1e-04 -8.28
14285 RP11-395N3.2 2_133 0.568 39.51 6.7e-05 6.44
3504 RAB29 1_104 0.563 21.24 3.6e-05 4.01
4222 OPA3 19_31 0.559 22.01 3.7e-05 3.96
5565 TACC2 10_76 0.521 25.32 3.9e-05 3.93Genes with largest effect sizes
#plot PIP vs effect size
plot(ctwas_gene_res$susie_pip, ctwas_gene_res$mu2, xlab="PIP", ylab="mu^2", main="Gene PIPs vs Effect Size")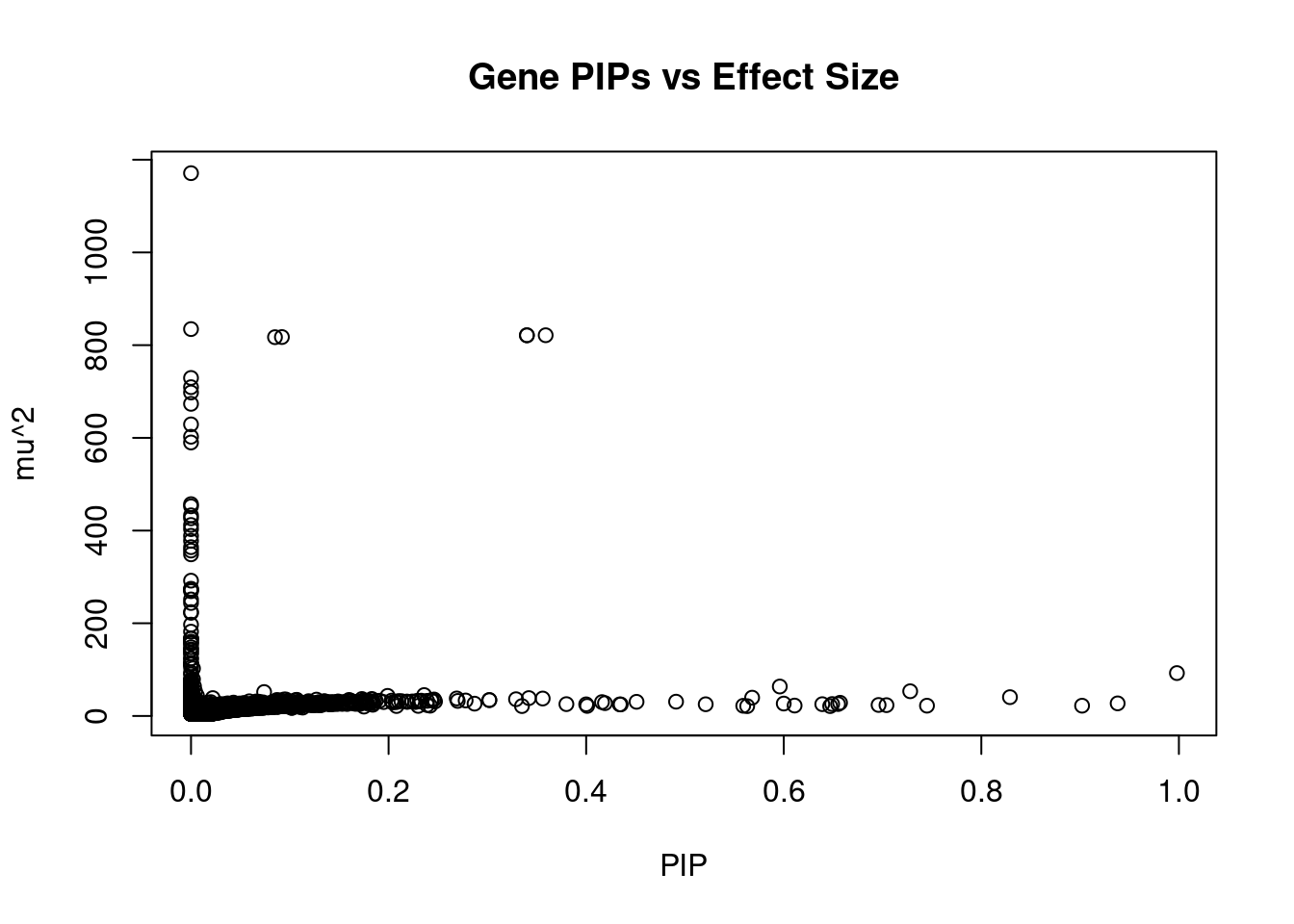
| Version | Author | Date |
|---|---|---|
| 3a7fbc1 | wesleycrouse | 2021-09-08 |
#genes with 20 largest effect sizes
head(ctwas_gene_res[order(-ctwas_gene_res$mu2),report_cols],20) genename region_tag susie_pip mu2 PVE z
6465 PPP1R18 6_24 0.000 1171.00 8.2e-15 5.18
12462 LINC00243 6_24 0.000 834.62 0.0e+00 -5.73
5257 THSD1 13_22 0.359 821.36 8.8e-04 2.37
5255 CKAP2 13_22 0.340 821.25 8.3e-04 2.37
14624 RP11-245D16.4 13_22 0.340 821.25 8.3e-04 -2.37
14412 RP11-93H24.3 13_22 0.092 817.45 2.2e-04 -2.33
8381 SUGT1 13_22 0.085 817.19 2.1e-04 2.33
13035 HLA-DQA2 6_26 0.000 729.22 0.0e+00 -9.41
11626 NEK5 13_22 0.000 709.34 9.3e-07 -2.22
12079 AGER 6_26 0.000 697.81 0.0e+00 -7.59
11608 ZSCAN26 6_22 0.000 673.37 0.0e+00 3.70
13457 UTP14C 13_22 0.000 629.07 1.6e-12 -1.56
8537 C2 6_26 0.000 602.40 0.0e+00 -8.14
11567 ZSCAN16 6_22 0.000 590.01 0.0e+00 2.16
5430 FLOT1 6_24 0.000 457.00 0.0e+00 -4.48
12080 RNF5 6_26 0.000 452.29 0.0e+00 6.17
12077 NOTCH4 6_26 0.000 432.86 0.0e+00 6.69
11407 ZKSCAN3 6_22 0.000 426.50 0.0e+00 2.62
12083 PRRT1 6_26 0.000 412.05 0.0e+00 -7.37
11236 ZSCAN23 6_22 0.000 403.10 0.0e+00 -2.34Genes with highest PVE
#genes with 20 highest pve
head(ctwas_gene_res[order(-ctwas_gene_res$PVE),report_cols],20) genename region_tag susie_pip mu2 PVE z
5257 THSD1 13_22 0.359 821.36 8.8e-04 2.37
5255 CKAP2 13_22 0.340 821.25 8.3e-04 2.37
14624 RP11-245D16.4 13_22 0.340 821.25 8.3e-04 -2.37
3633 CCND2 12_4 0.998 92.56 2.7e-04 9.86
14412 RP11-93H24.3 13_22 0.092 817.45 2.2e-04 -2.33
8381 SUGT1 13_22 0.085 817.19 2.1e-04 2.33
7048 JAZF1 7_23 0.728 53.35 1.2e-04 7.66
2723 WFS1 4_7 0.596 63.57 1.1e-04 -8.28
356 ANK1 8_36 0.829 40.62 1.0e-04 -6.50
13893 GTF2I 7_48 0.938 27.22 7.6e-05 -5.11
14285 RP11-395N3.2 2_133 0.568 39.51 6.7e-05 6.44
7923 INHBB 2_70 0.902 22.20 5.9e-05 -4.47
152 TFAP2B 6_38 0.657 28.53 5.6e-05 -5.10
7109 LONRF1 8_15 0.655 25.77 5.0e-05 4.08
3647 CCDC92 12_75 0.649 25.81 5.0e-05 4.43
7067 NUS1 6_78 0.745 22.23 4.9e-05 4.44
7749 C16orf59 16_2 0.696 23.77 4.9e-05 -3.88
1866 SLCO4A1 20_37 0.704 23.37 4.9e-05 -4.30
9518 MANEA 6_65 0.600 26.82 4.8e-05 3.99
11487 ZNF34 8_94 0.639 25.48 4.8e-05 -4.72Genes with largest z scores
#genes with 20 largest z scores
head(ctwas_gene_res[order(-abs(ctwas_gene_res$z)),report_cols],20) genename region_tag susie_pip mu2 PVE z
3633 CCND2 12_4 0.998 92.56 2.7e-04 9.86
13035 HLA-DQA2 6_26 0.000 729.22 0.0e+00 -9.41
2723 WFS1 4_7 0.596 63.57 1.1e-04 -8.28
8537 C2 6_26 0.000 602.40 0.0e+00 -8.14
7048 JAZF1 7_23 0.728 53.35 1.2e-04 7.66
12079 AGER 6_26 0.000 697.81 0.0e+00 -7.59
12097 NEU1 6_26 0.000 348.69 0.0e+00 -7.54
12083 PRRT1 6_26 0.000 412.05 0.0e+00 -7.37
12077 NOTCH4 6_26 0.000 432.86 0.0e+00 6.69
12371 CLIC1 6_26 0.000 364.16 0.0e+00 6.63
12101 VWA7 6_26 0.000 271.94 0.0e+00 6.54
12110 APOM 6_26 0.000 356.83 0.0e+00 6.52
356 ANK1 8_36 0.829 40.62 1.0e-04 -6.50
14285 RP11-395N3.2 2_133 0.568 39.51 6.7e-05 6.44
11430 HLA-DRB1 6_26 0.000 160.48 0.0e+00 6.44
12108 GPANK1 6_26 0.000 224.17 0.0e+00 6.37
12123 CCHCR1 6_26 0.000 167.14 0.0e+00 -6.25
12080 RNF5 6_26 0.000 452.29 0.0e+00 6.17
12075 HLA-DRA 6_26 0.000 251.42 0.0e+00 -5.97
12114 AIF1 6_26 0.000 273.21 0.0e+00 -5.93Comparing z scores and PIPs
#set nominal signifiance threshold for z scores
alpha <- 0.05
#bonferroni adjusted threshold for z scores
sig_thresh <- qnorm(1-(alpha/nrow(ctwas_gene_res)/2), lower=T)
#Q-Q plot for z scores
obs_z <- ctwas_gene_res$z[order(ctwas_gene_res$z)]
exp_z <- qnorm((1:nrow(ctwas_gene_res))/nrow(ctwas_gene_res))
plot(exp_z, obs_z, xlab="Expected z", ylab="Observed z", main="Gene z score Q-Q plot")
abline(a=0,b=1)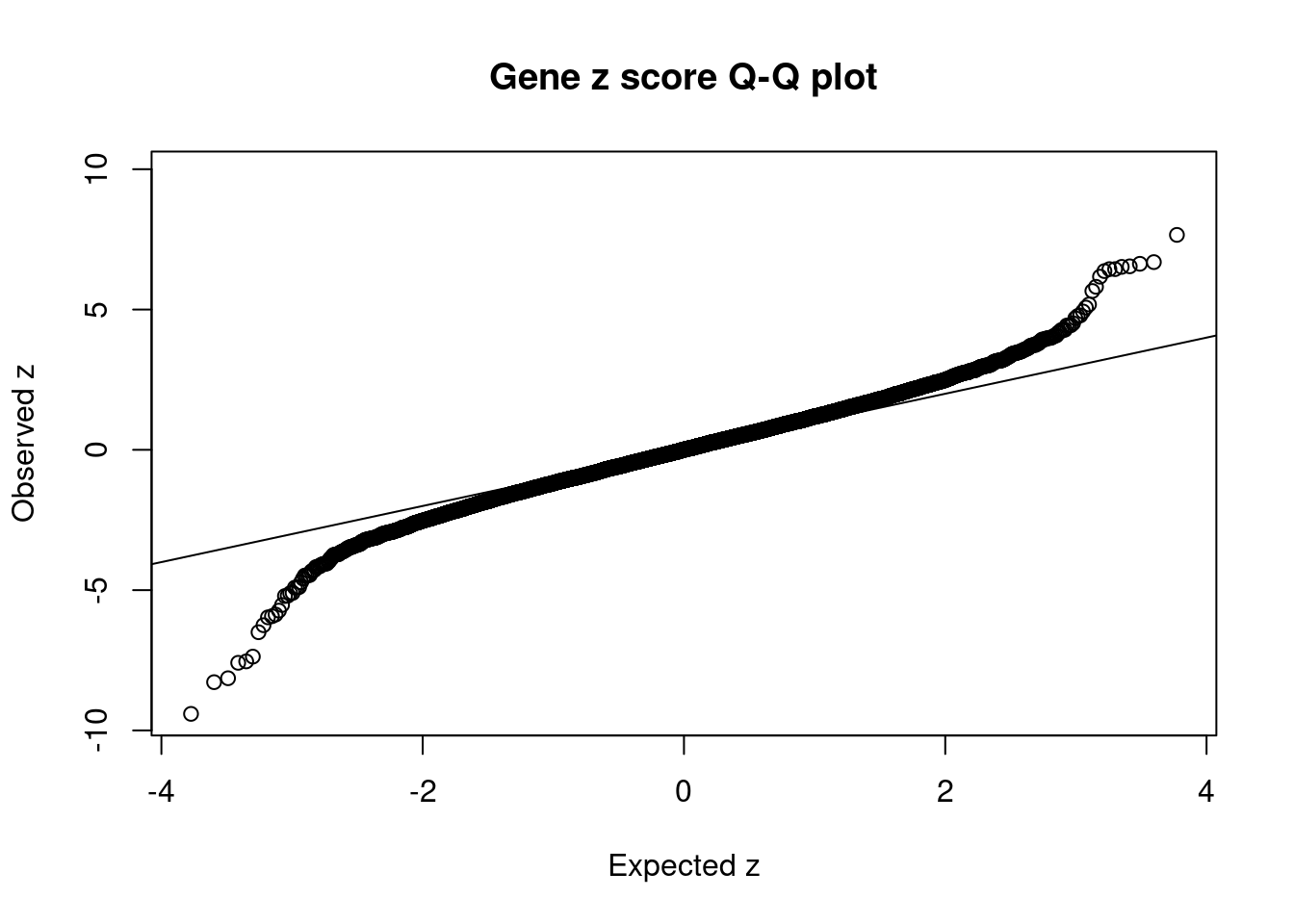
| Version | Author | Date |
|---|---|---|
| 3a7fbc1 | wesleycrouse | 2021-09-08 |
#plot z score vs PIP
plot(abs(ctwas_gene_res$z), ctwas_gene_res$susie_pip, xlab="abs(z)", ylab="PIP")
abline(v=sig_thresh, col="red", lty=2)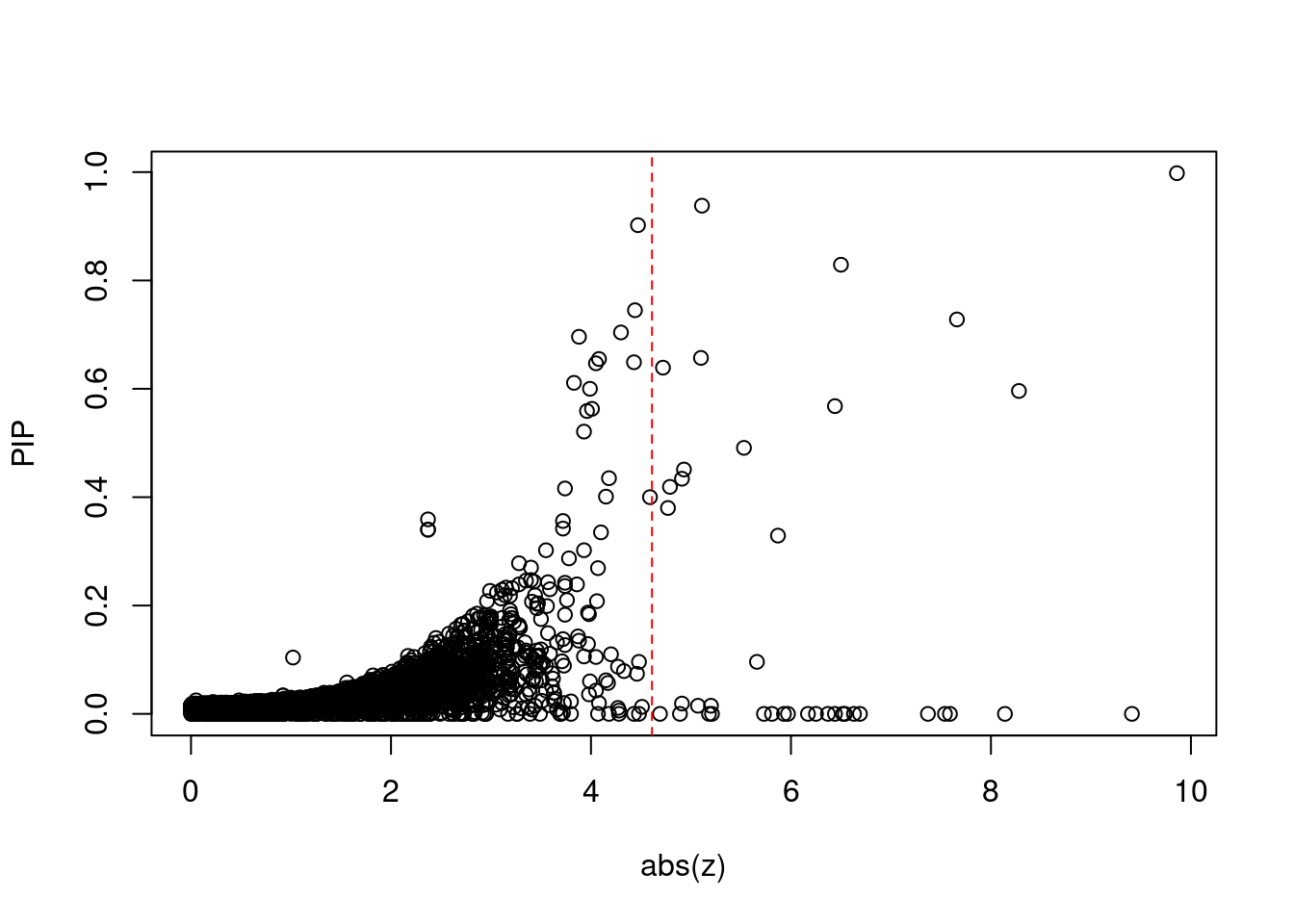
| Version | Author | Date |
|---|---|---|
| 3a7fbc1 | wesleycrouse | 2021-09-08 |
#proportion of significant z scores
mean(abs(ctwas_gene_res$z) > sig_thresh)[1] 0.003141614#genes with most significant z scores
head(ctwas_gene_res[order(-abs(ctwas_gene_res$z)),report_cols],20) genename region_tag susie_pip mu2 PVE z
3633 CCND2 12_4 0.998 92.56 2.7e-04 9.86
13035 HLA-DQA2 6_26 0.000 729.22 0.0e+00 -9.41
2723 WFS1 4_7 0.596 63.57 1.1e-04 -8.28
8537 C2 6_26 0.000 602.40 0.0e+00 -8.14
7048 JAZF1 7_23 0.728 53.35 1.2e-04 7.66
12079 AGER 6_26 0.000 697.81 0.0e+00 -7.59
12097 NEU1 6_26 0.000 348.69 0.0e+00 -7.54
12083 PRRT1 6_26 0.000 412.05 0.0e+00 -7.37
12077 NOTCH4 6_26 0.000 432.86 0.0e+00 6.69
12371 CLIC1 6_26 0.000 364.16 0.0e+00 6.63
12101 VWA7 6_26 0.000 271.94 0.0e+00 6.54
12110 APOM 6_26 0.000 356.83 0.0e+00 6.52
356 ANK1 8_36 0.829 40.62 1.0e-04 -6.50
14285 RP11-395N3.2 2_133 0.568 39.51 6.7e-05 6.44
11430 HLA-DRB1 6_26 0.000 160.48 0.0e+00 6.44
12108 GPANK1 6_26 0.000 224.17 0.0e+00 6.37
12123 CCHCR1 6_26 0.000 167.14 0.0e+00 -6.25
12080 RNF5 6_26 0.000 452.29 0.0e+00 6.17
12075 HLA-DRA 6_26 0.000 251.42 0.0e+00 -5.97
12114 AIF1 6_26 0.000 273.21 0.0e+00 -5.93Locus plots for genes and SNPs
ctwas_gene_res_sortz <- ctwas_gene_res[order(-abs(ctwas_gene_res$z)),]
n_plots <- 5
for (region_tag_plot in head(unique(ctwas_gene_res_sortz$region_tag), n_plots)){
ctwas_res_region <- ctwas_res[ctwas_res$region_tag==region_tag_plot,]
start <- min(ctwas_res_region$pos)
end <- max(ctwas_res_region$pos)
ctwas_res_region <- ctwas_res_region[order(ctwas_res_region$pos),]
ctwas_res_region_gene <- ctwas_res_region[ctwas_res_region$type=="gene",]
ctwas_res_region_snp <- ctwas_res_region[ctwas_res_region$type=="SNP",]
#region name
print(paste0("Region: ", region_tag_plot))
#table of genes in region
print(ctwas_res_region_gene[,report_cols])
par(mfrow=c(4,1))
#gene z scores
plot(ctwas_res_region_gene$pos, abs(ctwas_res_region_gene$z), xlab="Position", ylab="abs(gene_z)", xlim=c(start,end),
ylim=c(0,max(sig_thresh, abs(ctwas_res_region_gene$z))),
main=paste0("Region: ", region_tag_plot))
abline(h=sig_thresh,col="red",lty=2)
#significance threshold for SNPs
alpha_snp <- 5*10^(-8)
sig_thresh_snp <- qnorm(1-alpha_snp/2, lower=T)
#snp z scores
plot(ctwas_res_region_snp$pos, abs(ctwas_res_region_snp$z), xlab="Position", ylab="abs(snp_z)",xlim=c(start,end),
ylim=c(0,max(sig_thresh_snp, max(abs(ctwas_res_region_snp$z)))))
abline(h=sig_thresh_snp,col="purple",lty=2)
#gene pips
plot(ctwas_res_region_gene$pos, ctwas_res_region_gene$susie_pip, xlab="Position", ylab="Gene PIP", xlim=c(start,end), ylim=c(0,1))
abline(h=0.8,col="blue",lty=2)
#snp pips
plot(ctwas_res_region_snp$pos, ctwas_res_region_snp$susie_pip, xlab="Position", ylab="SNP PIP", xlim=c(start,end), ylim=c(0,1))
abline(h=0.8,col="blue",lty=2)
}[1] "Region: 12_4"
genename region_tag susie_pip mu2 PVE z
4562 CRACR2A 12_4 0.016 13.85 6.7e-07 1.45
2871 PARP11 12_4 0.023 17.18 1.1e-06 -1.88
3633 CCND2 12_4 0.998 92.56 2.7e-04 9.86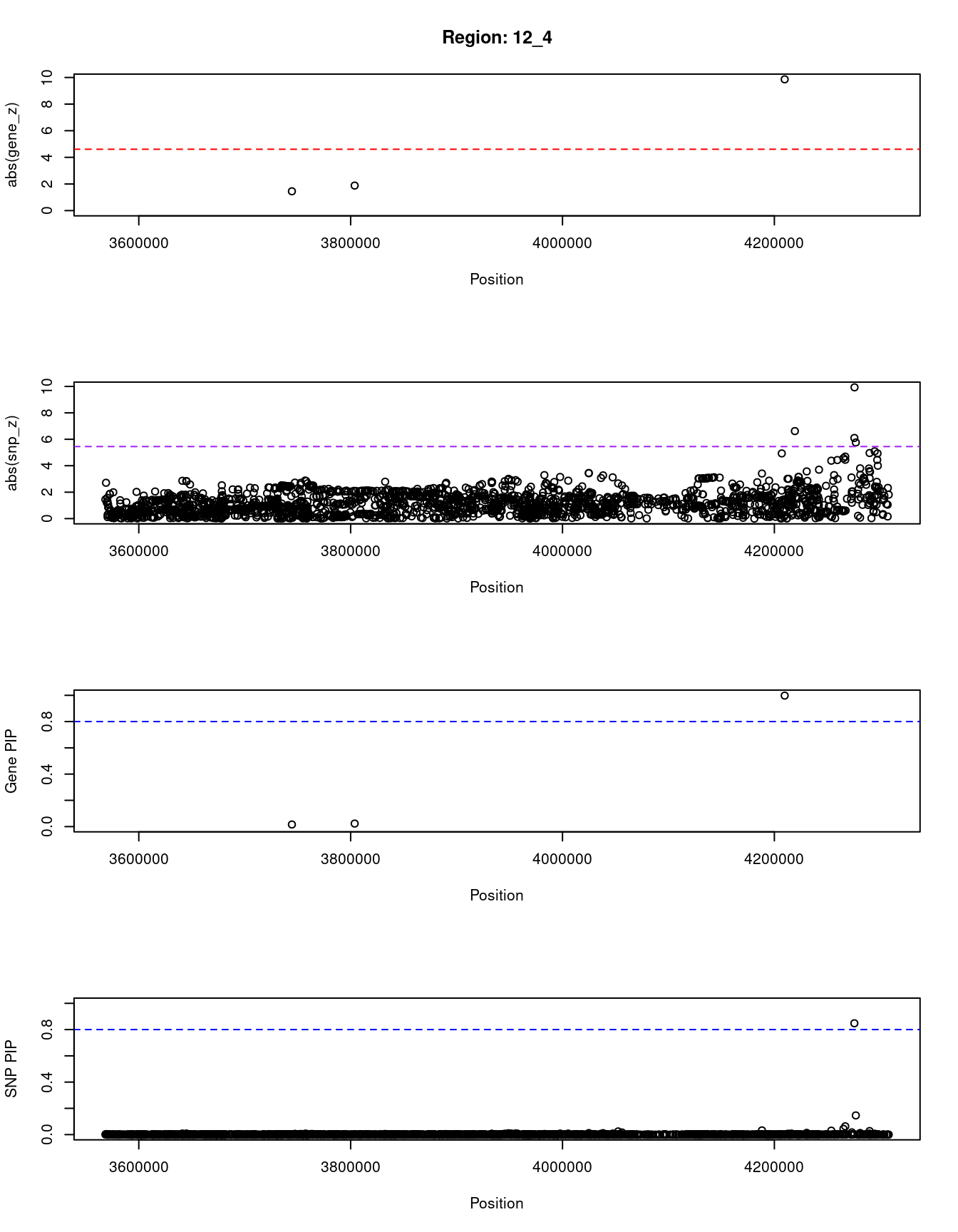
| Version | Author | Date |
|---|---|---|
| 3a7fbc1 | wesleycrouse | 2021-09-08 |
[1] "Region: 6_26"
genename region_tag susie_pip mu2 PVE z
12132 DDR1 6_26 0 10.92 0 -1.24
11458 SFTA2 6_26 0 26.40 0 2.29
12376 GTF2H4 6_26 0 8.12 0 0.93
5440 VARS2 6_26 0 6.11 0 0.61
12753 HCG22 6_26 0 24.16 0 2.17
12122 POU5F1 6_26 0 21.85 0 -2.04
12125 PSORS1C1 6_26 0 4.69 0 0.18
12124 PSORS1C2 6_26 0 5.08 0 -0.35
5429 TCF19 6_26 0 73.35 0 -4.07
12123 CCHCR1 6_26 0 167.14 0 -6.25
12277 HCG27 6_26 0 26.58 0 -2.30
12121 HLA-C 6_26 0 25.27 0 2.23
12942 HLA-B 6_26 0 9.61 0 1.10
14238 XXbac-BPG181B23.7 6_26 0 28.43 0 2.39
12120 MICA 6_26 0 40.20 0 -2.93
12119 MICB 6_26 0 77.25 0 -4.18
11873 DDX39B 6_26 0 18.39 0 -1.82
12374 ATP6V1G2 6_26 0 4.65 0 0.15
12117 NFKBIL1 6_26 0 13.25 0 -1.44
12871 TNF 6_26 0 23.28 0 2.12
12682 LTA 6_26 0 16.53 0 1.70
12115 NCR3 6_26 0 8.70 0 1.00
12090 DXO 6_26 0 33.95 0 3.49
12112 BAG6 6_26 0 11.03 0 -0.60
12114 AIF1 6_26 0 273.21 0 -5.93
12110 APOM 6_26 0 356.83 0 6.52
12109 C6orf47 6_26 0 31.95 0 -1.95
12108 GPANK1 6_26 0 224.17 0 6.37
12107 CSNK2B 6_26 0 57.14 0 -1.08
13093 LY6G5B 6_26 0 31.32 0 0.08
12105 ABHD16A 6_26 0 9.45 0 -1.59
12103 MPIG6B 6_26 0 34.42 0 -2.22
12104 LY6G6C 6_26 0 36.35 0 -0.01
12372 DDAH2 6_26 0 113.62 0 4.43
12102 MSH5 6_26 0 155.90 0 5.81
12371 CLIC1 6_26 0 364.16 0 6.63
12101 VWA7 6_26 0 271.94 0 6.54
12100 VARS 6_26 0 76.28 0 -3.39
12099 LSM2 6_26 0 24.61 0 -0.53
12098 C6orf48 6_26 0 31.04 0 -0.69
12097 NEU1 6_26 0 348.69 0 -7.54
12096 SLC44A4 6_26 0 76.94 0 -3.69
12094 EHMT2 6_26 0 9.67 0 2.63
13193 CFB 6_26 0 62.11 0 2.81
8537 C2 6_26 0 602.40 0 -8.14
12092 NELFE 6_26 0 57.82 0 -3.26
12091 SKIV2L 6_26 0 18.78 0 2.70
13236 C4A 6_26 0 222.51 0 4.69
12580 C4B 6_26 0 113.30 0 -5.21
12366 ATF6B 6_26 0 60.95 0 -4.89
12084 FKBPL 6_26 0 51.45 0 -2.01
12083 PRRT1 6_26 0 412.05 0 -7.37
12547 PPT2 6_26 0 30.28 0 1.63
13128 EGFL8 6_26 0 17.94 0 2.28
12080 RNF5 6_26 0 452.29 0 6.17
12081 AGPAT1 6_26 0 10.76 0 -2.18
12079 AGER 6_26 0 697.81 0 -7.59
12078 PBX2 6_26 0 59.97 0 2.95
12077 NOTCH4 6_26 0 432.86 0 6.69
12093 ZBTB12 6_26 0 39.38 0 -0.22
12075 HLA-DRA 6_26 0 251.42 0 -5.97
13035 HLA-DQA2 6_26 0 729.22 0 -9.41
11430 HLA-DRB1 6_26 0 160.48 0 6.44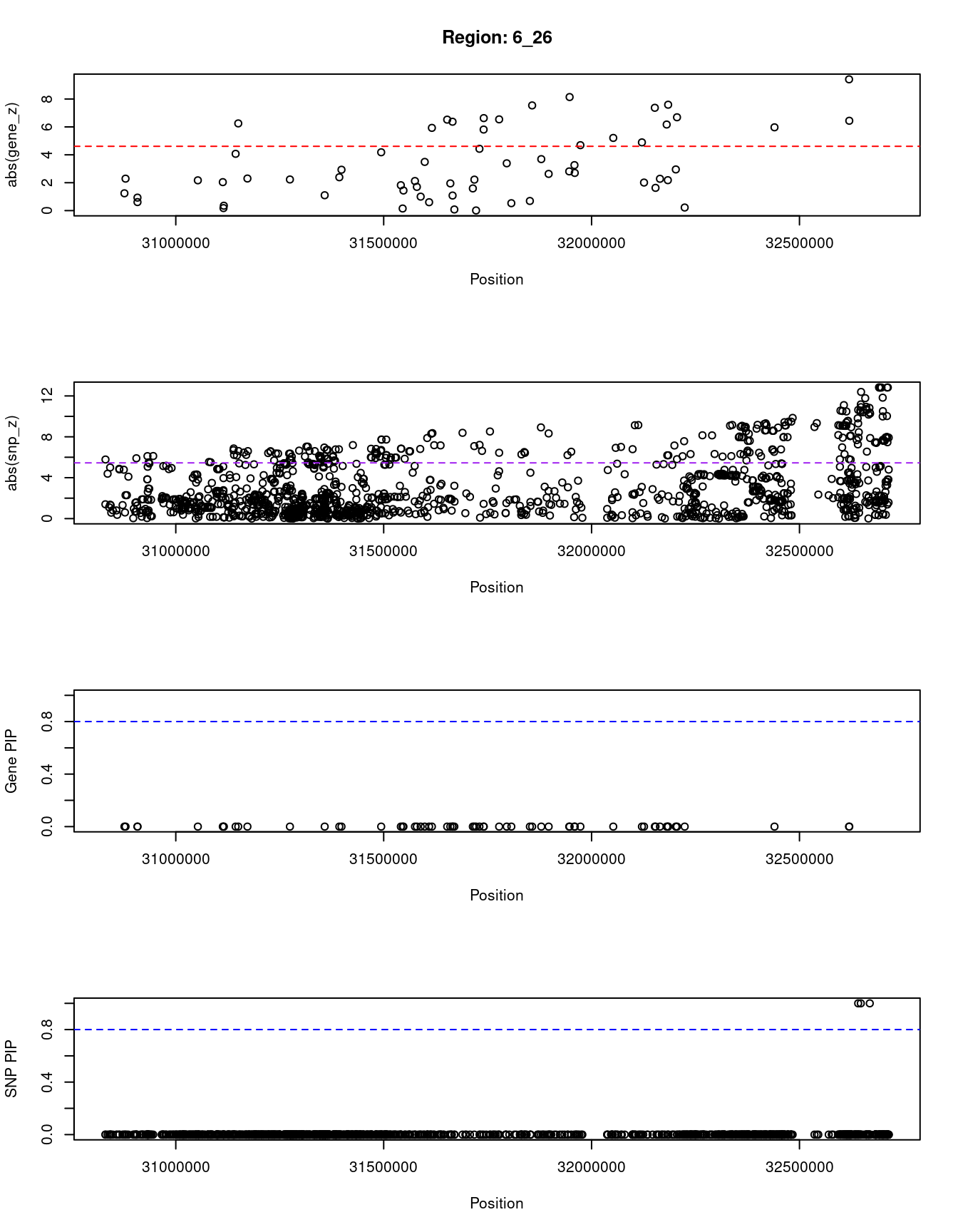
| Version | Author | Date |
|---|---|---|
| 3a7fbc1 | wesleycrouse | 2021-09-08 |
[1] "Region: 4_7"
genename region_tag susie_pip mu2 PVE z
9598 EVC2 4_7 0.015 8.38 3.9e-07 1.04
892 EVC 4_7 0.072 22.37 4.8e-06 2.15
891 CRMP1 4_7 0.010 4.57 1.4e-07 -0.04
6992 JAKMIP1 4_7 0.011 5.55 1.9e-07 -0.45
2723 WFS1 4_7 0.596 63.57 1.1e-04 -8.28
241 MAN2B2 4_7 0.010 4.57 1.4e-07 -0.04
10253 MRFAP1 4_7 0.020 10.46 6.1e-07 -1.24
9279 AC093323.3 4_7 0.020 10.71 6.5e-07 -1.17
13412 RP11-539L10.3 4_7 0.011 4.87 1.5e-07 -0.25
13269 RP11-539L10.2 4_7 0.010 4.60 1.4e-07 -0.10
8116 S100P 4_7 0.011 4.96 1.6e-07 -0.37
10250 MRFAP1L1 4_7 0.119 27.27 9.6e-06 2.61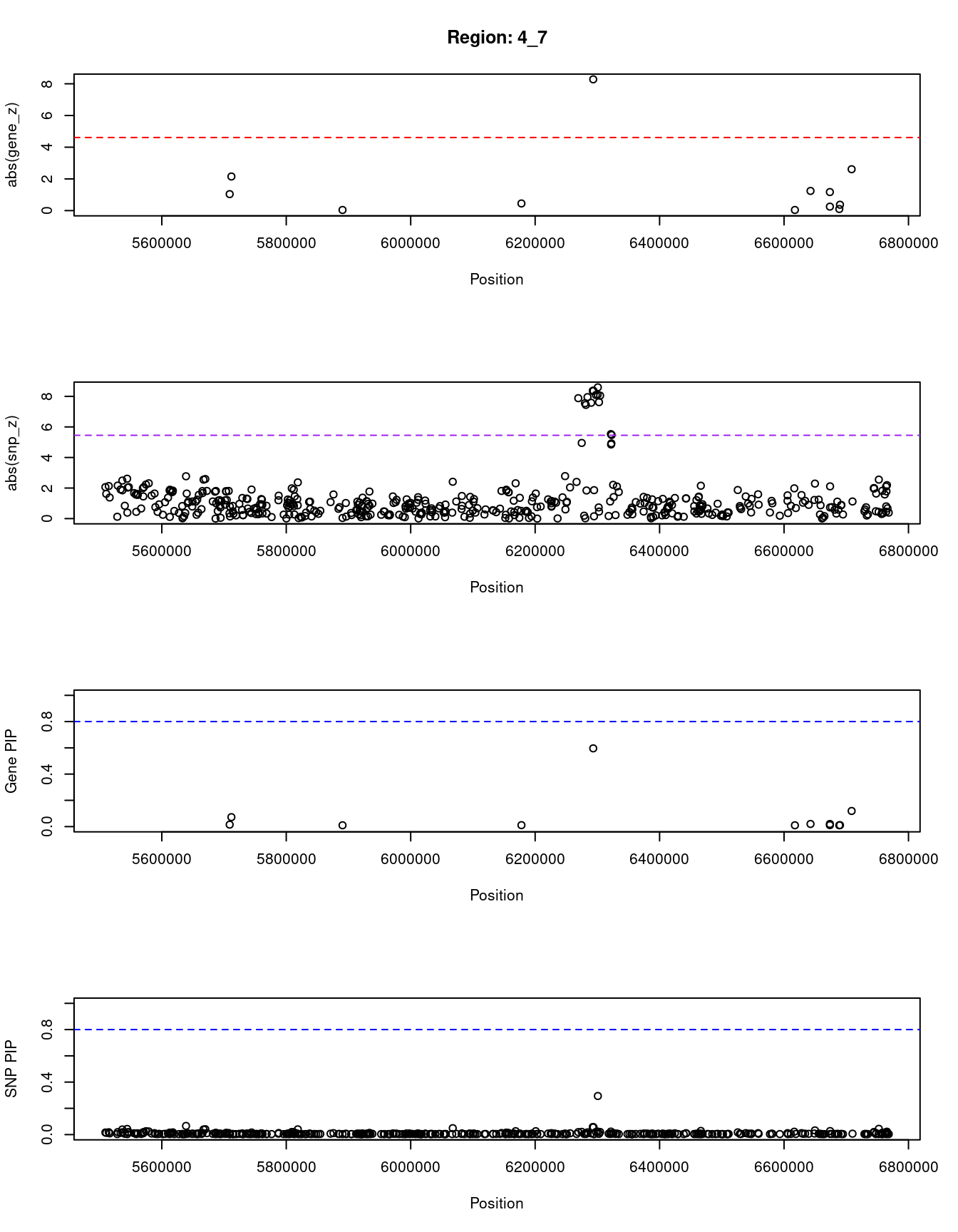
| Version | Author | Date |
|---|---|---|
| 3a7fbc1 | wesleycrouse | 2021-09-08 |
[1] "Region: 7_23"
genename region_tag susie_pip mu2 PVE z
14139 CTD-2227E11.1 7_23 0.008 6.17 1.4e-07 0.57
496 NFE2L3 7_23 0.006 4.57 8.6e-08 0.05
1262 SNX10 7_23 0.056 24.27 4.0e-06 2.50
3929 CBX3 7_23 0.056 24.27 4.0e-06 2.50
3926 KIAA0087 7_23 0.047 22.63 3.2e-06 2.26
12460 AC004540.5 7_23 0.047 22.63 3.2e-06 2.26
12902 AC004947.2 7_23 0.009 8.64 2.4e-07 1.34
2392 HOXA2 7_23 0.009 7.55 2.0e-07 -0.70
11693 HOXA4 7_23 0.006 4.58 8.7e-08 0.05
2395 HOXA5 7_23 0.007 5.68 1.2e-07 -0.32
2396 HOXA6 7_23 0.008 6.16 1.4e-07 -0.46
3933 HOXA7 7_23 0.007 5.00 1.0e-07 -0.16
1058 HOXA9 7_23 0.008 6.40 1.5e-07 0.54
55 HOXA11 7_23 0.016 12.99 6.3e-07 -1.40
2402 HIBADH 7_23 0.007 5.28 1.1e-07 0.19
2403 TAX1BP1 7_23 0.007 5.90 1.3e-07 1.02
7048 JAZF1 7_23 0.728 53.35 1.2e-04 7.66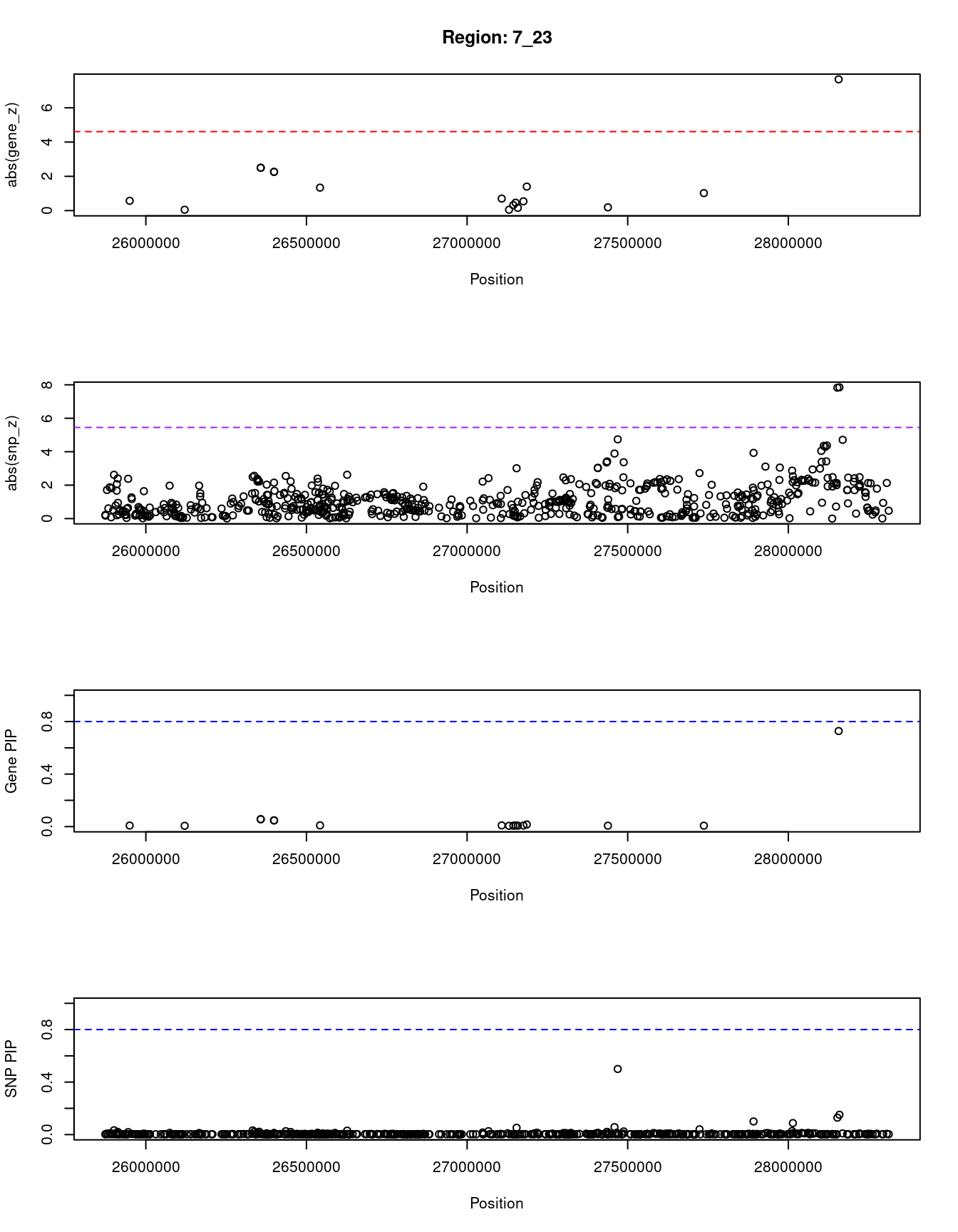
| Version | Author | Date |
|---|---|---|
| 3a7fbc1 | wesleycrouse | 2021-09-08 |
[1] "Region: 8_36"
genename region_tag susie_pip mu2 PVE z
8340 ZMAT4 8_36 0.020 10.82 6.4e-07 1.17
2134 SFRP1 8_36 0.011 5.66 1.9e-07 -0.39
6549 GOLGA7 8_36 0.116 28.08 9.7e-06 3.05
6551 GINS4 8_36 0.020 19.91 1.2e-06 -3.73
7444 GPAT4 8_36 0.010 8.95 2.6e-07 -2.18
356 ANK1 8_36 0.829 40.62 1.0e-04 -6.50
13780 RP11-930P14.2 8_36 0.051 20.36 3.1e-06 -2.47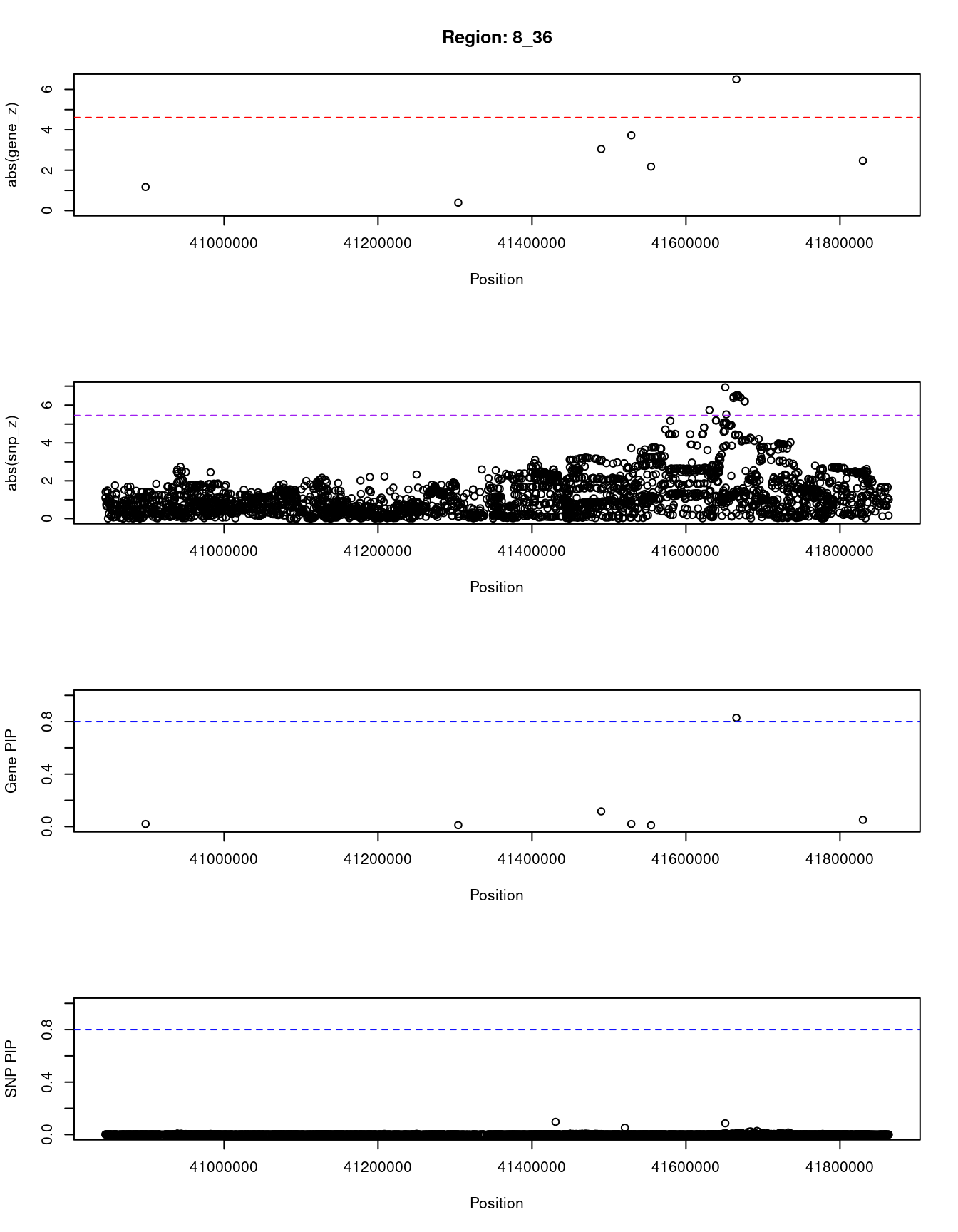
| Version | Author | Date |
|---|---|---|
| 3a7fbc1 | wesleycrouse | 2021-09-08 |
SNPs with highest PIPs
#snps with PIP>0.8 or 20 highest PIPs
head(ctwas_snp_res[order(-ctwas_snp_res$susie_pip),report_cols_snps],
max(sum(ctwas_snp_res$susie_pip>0.8), 20)) id region_tag susie_pip mu2 PVE z
5205 rs56219074 1_13 1.000 64.12 1.9e-04 2.18
37018 rs75222047 1_85 1.000 812.47 2.4e-03 2.91
42580 rs2776714 1_97 1.000 804.92 2.4e-03 -2.78
65949 rs780093 2_16 1.000 42.62 1.3e-04 6.98
82161 rs12471681 2_53 1.000 928.47 2.8e-03 -3.05
140234 rs13062973 3_45 1.000 616.64 1.8e-03 -3.60
168235 rs1388475 3_107 1.000 516.37 1.5e-03 2.87
168236 rs13071192 3_107 1.000 493.46 1.5e-03 -3.12
170701 rs28661518 3_112 1.000 984.19 2.9e-03 -3.09
210519 rs2125449 4_79 1.000 1030.47 3.1e-03 -3.33
253640 rs3910018 5_51 1.000 840.58 2.5e-03 -2.86
285039 rs2206734 6_15 1.000 84.17 2.5e-04 10.34
287337 rs34662244 6_22 1.000 1754.82 5.2e-03 4.07
287355 rs67297533 6_22 1.000 1718.45 5.1e-03 -4.09
289265 rs9272679 6_26 1.000 1758.27 5.2e-03 -9.31
289274 rs17612548 6_26 1.000 1832.90 5.4e-03 11.17
289295 rs3134996 6_26 1.000 1808.42 5.4e-03 10.37
289379 rs3916765 6_27 1.000 97.94 2.9e-04 10.72
380118 rs1495743 8_20 1.000 426.67 1.3e-03 3.21
407753 rs16902104 8_83 1.000 1289.53 3.8e-03 3.51
436626 rs7025746 9_53 1.000 393.97 1.2e-03 -3.04
436630 rs2900388 9_53 1.000 386.66 1.1e-03 3.51
472037 rs2019640 10_59 1.000 1037.47 3.1e-03 -3.47
472039 rs913647 10_59 1.000 1039.87 3.1e-03 3.45
477392 rs12244851 10_70 1.000 256.46 7.6e-04 22.39
484930 rs234856 11_2 1.000 37.78 1.1e-04 -4.94
486963 rs11041828 11_6 1.000 898.91 2.7e-03 2.96
486964 rs4237770 11_6 1.000 917.68 2.7e-03 -2.93
498903 rs113527193 11_30 1.000 455.70 1.4e-03 3.29
499234 rs11603701 11_30 1.000 464.24 1.4e-03 -3.24
567100 rs9526909 13_22 1.000 735.06 2.2e-03 2.54
614351 rs12912777 15_13 1.000 33.43 9.9e-05 5.53
650842 rs12443634 16_45 1.000 194.77 5.8e-04 -3.47
650849 rs2966092 16_45 1.000 197.33 5.9e-04 3.38
656370 rs4790233 17_5 1.000 204.73 6.1e-04 -3.70
656375 rs8072531 17_5 1.000 205.45 6.1e-04 -3.98
702027 rs73537429 19_15 1.000 284.83 8.5e-04 -2.52
721423 rs2747568 20_20 1.000 1342.76 4.0e-03 3.79
721424 rs2064505 20_20 1.000 1375.04 4.1e-03 -3.92
429944 rs9410573 9_40 0.999 47.20 1.4e-04 -7.44
663260 rs9906451 17_22 0.999 30.57 9.1e-05 -5.87
702056 rs2285626 19_15 0.997 121.43 3.6e-04 5.36
380121 rs34537991 8_20 0.995 369.75 1.1e-03 3.06
574920 rs1327315 13_40 0.994 32.66 9.7e-05 -7.40
421232 rs10965243 9_16 0.992 26.51 7.8e-05 -5.41
702029 rs67238925 19_15 0.991 275.79 8.1e-04 2.39
759997 rs3129696 6_24 0.990 1625.72 4.8e-03 -3.92
436629 rs7868850 9_53 0.987 75.39 2.2e-04 0.83
316978 rs138764591 6_89 0.982 24.34 7.1e-05 2.59
168238 rs13081434 3_107 0.980 473.40 1.4e-03 -2.47
289480 rs2857161 6_27 0.977 87.70 2.5e-04 6.21
436627 rs4742930 9_53 0.973 304.65 8.8e-04 3.25
47621 rs79687284 1_109 0.960 26.04 7.4e-05 5.66
484929 rs234852 11_2 0.954 26.07 7.4e-05 3.12
29222 rs6679677 1_70 0.949 26.06 7.3e-05 5.33
407752 rs16902103 8_83 0.942 1262.88 3.5e-03 -3.50
538201 rs189339 12_40 0.942 33.37 9.3e-05 -6.27
154541 rs72964564 3_76 0.932 33.29 9.2e-05 -6.10
472117 rs1977833 10_59 0.927 101.36 2.8e-04 -8.47
256835 rs10479168 5_59 0.918 26.05 7.1e-05 5.36
170702 rs73190822 3_112 0.917 962.93 2.6e-03 3.11
574932 rs502027 13_40 0.909 31.65 8.5e-05 7.22
171586 rs6444187 3_114 0.906 25.70 6.9e-05 5.17
205072 rs35518360 4_67 0.878 24.26 6.3e-05 4.70
648085 rs72802395 16_40 0.877 35.62 9.3e-05 -6.35
535250 rs2682323 12_33 0.873 24.09 6.2e-05 4.74
778230 rs3217792 12_4 0.848 37.47 9.4e-05 -6.10
37037 rs2179109 1_85 0.843 796.04 2.0e-03 -3.03
140222 rs34695932 3_45 0.826 644.14 1.6e-03 4.13
656366 rs79425343 17_5 0.826 25.04 6.1e-05 1.15
170700 rs62287225 3_112 0.820 962.71 2.3e-03 3.08
160081 rs73148854 3_89 0.809 23.36 5.6e-05 -4.35
139500 rs3774723 3_43 0.807 25.03 6.0e-05 -4.84
709084 rs12609461 19_33 0.807 29.07 7.0e-05 5.53SNPs with largest effect sizes
#plot PIP vs effect size
plot(ctwas_snp_res$susie_pip, ctwas_snp_res$mu2, xlab="PIP", ylab="mu^2", main="SNP PIPs vs Effect Size")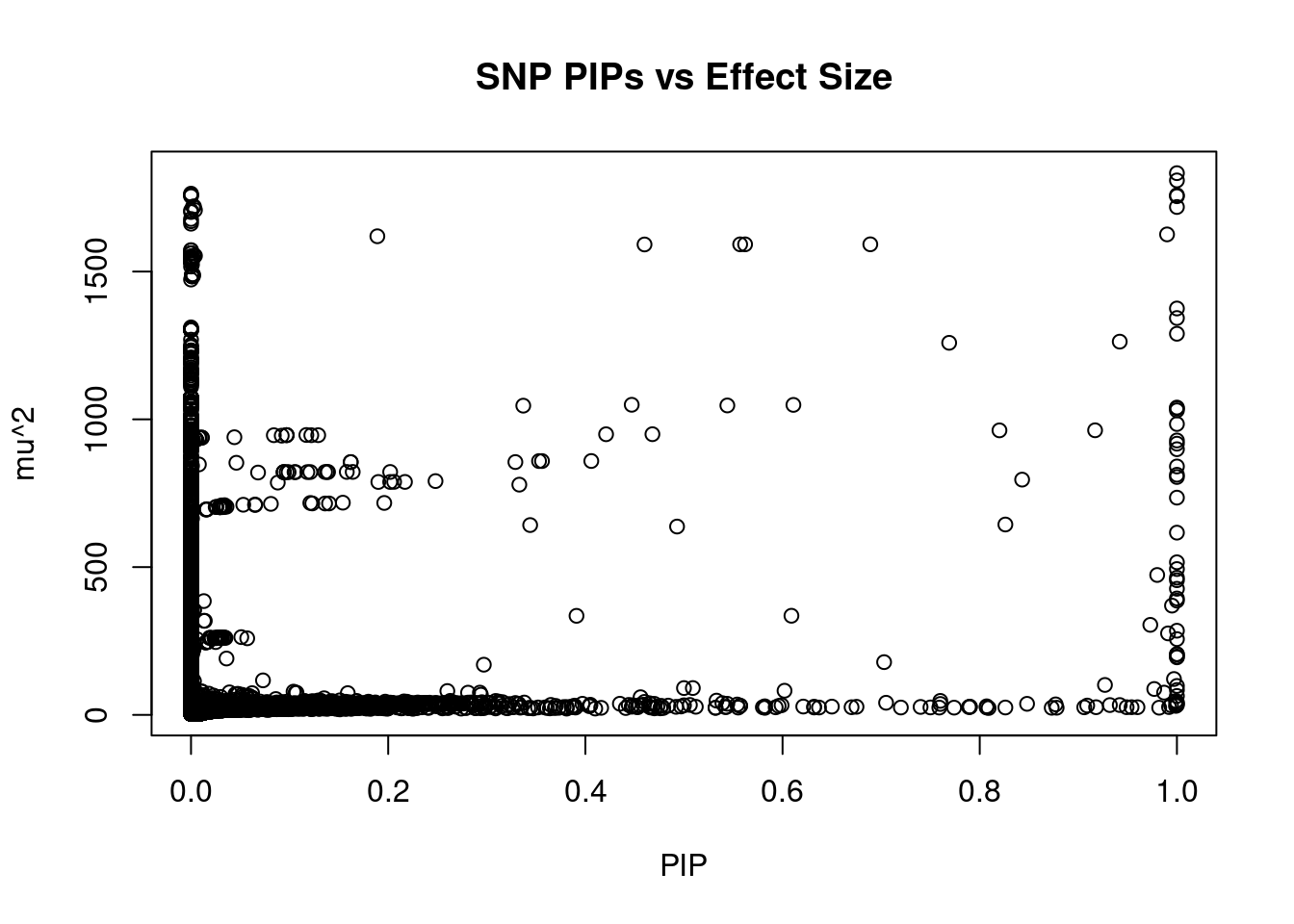
| Version | Author | Date |
|---|---|---|
| 3a7fbc1 | wesleycrouse | 2021-09-08 |
#SNPs with 50 largest effect sizes
head(ctwas_snp_res[order(-ctwas_snp_res$mu2),report_cols_snps],50) id region_tag susie_pip mu2 PVE z
289274 rs17612548 6_26 1.000 1832.90 5.4e-03 11.17
289295 rs3134996 6_26 1.000 1808.42 5.4e-03 10.37
289273 rs17612510 6_26 0.000 1763.56 1.7e-08 10.42
289281 rs9273524 6_26 0.000 1759.68 0.0e+00 10.93
289275 rs17612633 6_26 0.000 1758.58 0.0e+00 10.90
289265 rs9272679 6_26 1.000 1758.27 5.2e-03 -9.31
287337 rs34662244 6_22 1.000 1754.82 5.2e-03 4.07
289272 rs9273242 6_26 0.000 1754.81 0.0e+00 10.58
287392 rs13191786 6_22 0.003 1719.94 1.3e-05 4.10
287395 rs34218844 6_22 0.002 1719.69 1.1e-05 4.09
287355 rs67297533 6_22 1.000 1718.45 5.1e-03 -4.09
287381 rs67998226 6_22 0.004 1708.00 2.0e-05 4.25
287400 rs35030260 6_22 0.000 1706.52 1.3e-06 4.12
287386 rs33932084 6_22 0.000 1701.37 1.2e-08 3.93
287405 rs13198809 6_22 0.000 1677.89 1.3e-09 4.08
287414 rs36092177 6_22 0.000 1670.16 2.2e-10 4.08
287439 rs55690788 6_22 0.000 1661.87 1.8e-10 4.16
759997 rs3129696 6_24 0.990 1625.72 4.8e-03 -3.92
759989 rs2022083 6_24 0.189 1619.39 9.1e-04 -3.89
759944 rs2844773 6_24 0.689 1592.18 3.3e-03 3.95
759961 rs2188099 6_24 0.557 1592.09 2.6e-03 3.92
759965 rs2844770 6_24 0.562 1592.09 2.7e-03 3.92
759949 rs2844771 6_24 0.460 1591.64 2.2e-03 3.91
287551 rs1311917 6_22 0.000 1572.58 0.0e+00 3.82
287543 rs1233611 6_22 0.000 1571.53 0.0e+00 3.78
287561 rs9257192 6_22 0.000 1562.49 0.0e+00 3.92
287575 rs3135297 6_22 0.000 1561.62 0.0e+00 3.93
287580 rs9257267 6_22 0.000 1561.39 0.0e+00 3.90
760042 rs3094076 6_24 0.003 1553.73 1.6e-05 3.81
760110 rs3094629 6_24 0.004 1553.42 1.7e-05 3.81
760120 rs3129704 6_24 0.003 1552.91 1.2e-05 3.79
287597 rs3130838 6_22 0.000 1551.54 0.0e+00 3.95
760037 rs3094632 6_24 0.000 1546.87 1.4e-06 3.68
760040 rs3094077 6_24 0.000 1545.30 1.4e-06 3.69
760124 rs3129836 6_24 0.002 1545.21 1.0e-05 3.83
760121 rs3129705 6_24 0.000 1543.51 1.4e-06 3.70
760128 rs3129707 6_24 0.000 1543.22 1.0e-06 3.69
760085 rs3130398 6_24 0.000 1543.19 9.8e-07 3.68
760095 rs3130402 6_24 0.000 1543.19 9.7e-07 3.68
760097 rs9261636 6_24 0.000 1543.18 9.6e-07 3.68
760103 rs3130404 6_24 0.000 1543.17 9.5e-07 3.68
760113 rs3132656 6_24 0.000 1543.17 9.5e-07 3.68
760114 rs3132655 6_24 0.000 1543.17 9.5e-07 3.68
760049 rs3129697 6_24 0.000 1543.16 1.0e-06 3.69
760088 rs3129700 6_24 0.000 1543.16 9.4e-07 3.68
760093 rs3130401 6_24 0.000 1543.16 9.5e-07 3.68
760076 rs3129699 6_24 0.000 1543.15 9.5e-07 3.68
760115 rs3130405 6_24 0.000 1543.14 9.3e-07 3.68
760098 rs3132658 6_24 0.000 1543.13 9.2e-07 3.68
760092 rs3094073 6_24 0.000 1543.04 9.5e-07 3.68SNPs with highest PVE
#SNPs with 50 highest pve
head(ctwas_snp_res[order(-ctwas_snp_res$PVE),report_cols_snps],50) id region_tag susie_pip mu2 PVE z
289274 rs17612548 6_26 1.000 1832.90 0.00540 11.17
289295 rs3134996 6_26 1.000 1808.42 0.00540 10.37
287337 rs34662244 6_22 1.000 1754.82 0.00520 4.07
289265 rs9272679 6_26 1.000 1758.27 0.00520 -9.31
287355 rs67297533 6_22 1.000 1718.45 0.00510 -4.09
759997 rs3129696 6_24 0.990 1625.72 0.00480 -3.92
721424 rs2064505 20_20 1.000 1375.04 0.00410 -3.92
721423 rs2747568 20_20 1.000 1342.76 0.00400 3.79
407753 rs16902104 8_83 1.000 1289.53 0.00380 3.51
407752 rs16902103 8_83 0.942 1262.88 0.00350 -3.50
759944 rs2844773 6_24 0.689 1592.18 0.00330 3.95
210519 rs2125449 4_79 1.000 1030.47 0.00310 -3.33
472037 rs2019640 10_59 1.000 1037.47 0.00310 -3.47
472039 rs913647 10_59 1.000 1039.87 0.00310 3.45
170701 rs28661518 3_112 1.000 984.19 0.00290 -3.09
407750 rs34585331 8_83 0.769 1258.92 0.00290 -3.55
82161 rs12471681 2_53 1.000 928.47 0.00280 -3.05
486963 rs11041828 11_6 1.000 898.91 0.00270 2.96
486964 rs4237770 11_6 1.000 917.68 0.00270 -2.93
759965 rs2844770 6_24 0.562 1592.09 0.00270 3.92
170702 rs73190822 3_112 0.917 962.93 0.00260 3.11
759961 rs2188099 6_24 0.557 1592.09 0.00260 3.92
253640 rs3910018 5_51 1.000 840.58 0.00250 -2.86
37018 rs75222047 1_85 1.000 812.47 0.00240 2.91
42580 rs2776714 1_97 1.000 804.92 0.00240 -2.78
170700 rs62287225 3_112 0.820 962.71 0.00230 3.08
567100 rs9526909 13_22 1.000 735.06 0.00220 2.54
759949 rs2844771 6_24 0.460 1591.64 0.00220 3.91
37037 rs2179109 1_85 0.843 796.04 0.00200 -3.03
210539 rs11721784 4_79 0.611 1048.87 0.00190 3.38
140234 rs13062973 3_45 1.000 616.64 0.00180 -3.60
210505 rs13109930 4_79 0.544 1046.98 0.00170 3.11
140222 rs34695932 3_45 0.826 644.14 0.00160 4.13
168235 rs1388475 3_107 1.000 516.37 0.00150 2.87
168236 rs13071192 3_107 1.000 493.46 0.00150 -3.12
168238 rs13081434 3_107 0.980 473.40 0.00140 -2.47
210526 rs1542437 4_79 0.447 1049.25 0.00140 3.35
498903 rs113527193 11_30 1.000 455.70 0.00140 3.29
499234 rs11603701 11_30 1.000 464.24 0.00140 -3.24
82157 rs75755471 2_53 0.468 949.72 0.00130 3.07
380118 rs1495743 8_20 1.000 426.67 0.00130 3.21
82163 rs78406274 2_53 0.421 949.54 0.00120 3.06
436626 rs7025746 9_53 1.000 393.97 0.00120 -3.04
380121 rs34537991 8_20 0.995 369.75 0.00110 3.06
436630 rs2900388 9_53 1.000 386.66 0.00110 3.51
210507 rs6819187 4_79 0.337 1046.26 0.00100 3.09
253651 rs10805847 5_51 0.406 859.00 0.00100 2.87
140228 rs13071831 3_45 0.493 637.26 0.00093 4.15
253645 rs3910025 5_51 0.356 858.84 0.00091 2.86
759989 rs2022083 6_24 0.189 1619.39 0.00091 -3.89SNPs with largest z scores
#SNPs with 50 largest z scores
head(ctwas_snp_res[order(-abs(ctwas_snp_res$z)),report_cols_snps],50) id region_tag susie_pip mu2 PVE z
477400 rs12260037 10_70 0.003 227.92 2.3e-06 22.65
477392 rs12244851 10_70 1.000 256.46 7.6e-04 22.39
477389 rs6585198 10_70 0.001 126.84 3.1e-07 15.56
477401 rs12359102 10_70 0.000 97.20 1.3e-07 14.81
477399 rs7077039 10_70 0.000 96.94 1.3e-07 14.79
477397 rs7921525 10_70 0.000 93.63 1.4e-07 14.58
477405 rs4077527 10_70 0.000 88.00 1.3e-07 14.57
477398 rs6585203 10_70 0.000 92.71 1.4e-07 14.52
477394 rs11196191 10_70 0.000 92.53 1.4e-07 14.51
477395 rs10787472 10_70 0.001 92.11 1.4e-07 14.49
289317 rs9275221 6_26 0.000 354.03 0.0e+00 12.84
289368 rs9275583 6_26 0.000 354.14 0.0e+00 12.83
289325 rs9275275 6_26 0.000 352.61 0.0e+00 12.82
289330 rs9275307 6_26 0.000 352.88 0.0e+00 12.82
289361 rs4273728 6_26 0.000 352.97 0.0e+00 12.82
289319 rs9275223 6_26 0.000 352.43 0.0e+00 12.81
289276 rs17612712 6_26 0.000 1251.80 0.0e+00 12.39
477383 rs12243578 10_70 0.002 80.12 3.8e-07 12.36
289337 rs9275362 6_26 0.000 182.28 0.0e+00 11.83
289278 rs9273354 6_26 0.000 1185.78 0.0e+00 11.78
289274 rs17612548 6_26 1.000 1832.90 5.4e-03 11.17
289197 rs617578 6_26 0.000 259.88 0.0e+00 11.10
289281 rs9273524 6_26 0.000 1759.68 0.0e+00 10.93
289275 rs17612633 6_26 0.000 1758.58 0.0e+00 10.90
289293 rs3828800 6_26 0.000 275.02 0.0e+00 10.83
289282 rs9273528 6_26 0.000 241.46 0.0e+00 10.73
289379 rs3916765 6_27 1.000 97.94 2.9e-04 10.72
289266 rs34763586 6_26 0.000 451.05 0.0e+00 10.63
289284 rs28724252 6_26 0.000 238.50 0.0e+00 10.61
289272 rs9273242 6_26 0.000 1754.81 0.0e+00 10.58
289185 rs2760985 6_26 0.000 423.92 0.0e+00 10.56
171280 rs11929397 3_114 0.509 90.84 1.4e-04 10.55
171281 rs11716713 3_114 0.500 90.79 1.3e-04 10.55
289191 rs2647066 6_26 0.000 423.44 0.0e+00 10.52
289340 rs3135002 6_26 0.000 1051.42 0.0e+00 10.51
289210 rs3997872 6_26 0.000 424.43 0.0e+00 10.48
289273 rs17612510 6_26 0.000 1763.56 1.7e-08 10.42
289277 rs17612852 6_26 0.000 1269.51 0.0e+00 10.40
289295 rs3134996 6_26 1.000 1808.42 5.4e-03 10.37
285039 rs2206734 6_15 1.000 84.17 2.5e-04 10.34
289296 rs3134994 6_26 0.000 1165.37 0.0e+00 10.28
289290 rs3830058 6_26 0.000 1156.66 0.0e+00 10.17
289359 rs3129726 6_26 0.000 1122.49 0.0e+00 10.03
289336 rs3135190 6_26 0.000 1137.44 0.0e+00 9.99
778231 rs76895963 12_4 0.002 80.69 5.1e-07 -9.93
289170 rs12191360 6_26 0.000 454.79 0.0e+00 9.85
289263 rs41269945 6_26 0.000 444.20 0.0e+00 9.81
289221 rs3129757 6_26 0.000 766.69 0.0e+00 9.56
642712 rs79994966 16_29 0.293 75.37 6.6e-05 9.56
642708 rs17817712 16_29 0.281 75.24 6.3e-05 9.55Gene set enrichment for genes with PIP>0.8
#GO enrichment analysis
library(enrichR)Welcome to enrichR
Checking connection ... Enrichr ... Connection is Live!
FlyEnrichr ... Connection is available!
WormEnrichr ... Connection is available!
YeastEnrichr ... Connection is available!
FishEnrichr ... Connection is available!dbs <- c("GO_Biological_Process_2021", "GO_Cellular_Component_2021", "GO_Molecular_Function_2021")
genes <- ctwas_gene_res$genename[ctwas_gene_res$susie_pip>0.8]
#number of genes for gene set enrichment
length(genes)[1] 4if (length(genes)>0){
GO_enrichment <- enrichr(genes, dbs)
for (db in dbs){
print(db)
df <- GO_enrichment[[db]]
df <- df[df$Adjusted.P.value<0.05,c("Term", "Overlap", "Adjusted.P.value", "Genes")]
print(df)
}
#DisGeNET enrichment
# devtools::install_bitbucket("ibi_group/disgenet2r")
library(disgenet2r)
disgenet_api_key <- get_disgenet_api_key(
email = "wesleycrouse@gmail.com",
password = "uchicago1" )
Sys.setenv(DISGENET_API_KEY= disgenet_api_key)
res_enrich <-disease_enrichment(entities=genes, vocabulary = "HGNC",
database = "CURATED" )
df <- res_enrich@qresult[1:10, c("Description", "FDR", "Ratio", "BgRatio")]
print(df)
#WebGestalt enrichment
library(WebGestaltR)
background <- ctwas_gene_res$genename
#listGeneSet()
databases <- c("pathway_KEGG", "disease_GLAD4U", "disease_OMIM")
enrichResult <- WebGestaltR(enrichMethod="ORA", organism="hsapiens",
interestGene=genes, referenceGene=background,
enrichDatabase=databases, interestGeneType="genesymbol",
referenceGeneType="genesymbol", isOutput=F)
print(enrichResult[,c("description", "size", "overlap", "FDR", "database", "userId")])
}Uploading data to Enrichr... Done.
Querying GO_Biological_Process_2021... Done.
Querying GO_Cellular_Component_2021... Done.
Querying GO_Molecular_Function_2021... Done.
Parsing results... Done.
[1] "GO_Biological_Process_2021"
Term
1 negative regulation of gonadotropin secretion (GO:0032277)
2 maintenance of apical/basal cell polarity (GO:0035090)
3 maintenance of epithelial cell apical/basal polarity (GO:0045199)
4 positive regulation of protein phosphorylation (GO:0001934)
5 ovarian follicle development (GO:0001541)
6 negative regulation of insulin secretion (GO:0046676)
7 positive regulation of cyclin-dependent protein serine/threonine kinase activity (GO:0045737)
8 negative regulation of peptide hormone secretion (GO:0090278)
9 activin receptor signaling pathway (GO:0032924)
10 positive regulation of cyclin-dependent protein kinase activity (GO:1904031)
11 female gonad development (GO:0008585)
12 positive regulation of G1/S transition of mitotic cell cycle (GO:1900087)
13 establishment or maintenance of epithelial cell apical/basal polarity (GO:0045197)
14 positive regulation of reproductive process (GO:2000243)
15 positive regulation of cell cycle G1/S phase transition (GO:1902808)
16 negative regulation of protein secretion (GO:0050709)
17 positive regulation of pathway-restricted SMAD protein phosphorylation (GO:0010862)
18 negative regulation of protein metabolic process (GO:0051248)
19 regulation of cyclin-dependent protein kinase activity (GO:1904029)
20 regulation of pathway-restricted SMAD protein phosphorylation (GO:0060393)
21 fat cell differentiation (GO:0045444)
22 positive regulation of mitotic cell cycle phase transition (GO:1901992)
23 cellular response to nutrient levels (GO:0031669)
24 positive regulation of cell cycle (GO:0045787)
25 regulation of G1/S transition of mitotic cell cycle (GO:2000045)
26 negative regulation of blood vessel morphogenesis (GO:2000181)
27 regulation of cyclin-dependent protein serine/threonine kinase activity (GO:0000079)
28 response to insulin (GO:0032868)
29 negative regulation of angiogenesis (GO:0016525)
30 positive regulation of transmembrane receptor protein serine/threonine kinase signaling pathway (GO:0090100)
31 regulation of insulin secretion (GO:0050796)
32 positive regulation of protein serine/threonine kinase activity (GO:0071902)
33 cellular response to peptide hormone stimulus (GO:0071375)
34 regulation of protein serine/threonine kinase activity (GO:0071900)
35 cytoskeleton organization (GO:0007010)
36 cellular response to insulin stimulus (GO:0032869)
37 transmembrane receptor protein serine/threonine kinase signaling pathway (GO:0007178)
38 protein localization to plasma membrane (GO:0072659)
39 protein localization to cell periphery (GO:1990778)
40 cellular response to starvation (GO:0009267)
41 negative regulation of cytokine production (GO:0001818)
42 endoplasmic reticulum to Golgi vesicle-mediated transport (GO:0006888)
Overlap Adjusted.P.value Genes
1 1/5 0.02276379 INHBB
2 1/9 0.02276379 ANK1
3 1/9 0.02276379 ANK1
4 2/371 0.02276379 CCND2;INHBB
5 1/14 0.02276379 INHBB
6 1/17 0.02276379 INHBB
7 1/17 0.02276379 CCND2
8 1/18 0.02276379 INHBB
9 1/19 0.02276379 INHBB
10 1/20 0.02276379 CCND2
11 1/22 0.02276379 INHBB
12 1/26 0.02465339 CCND2
13 1/32 0.02653193 ANK1
14 1/35 0.02653193 INHBB
15 1/35 0.02653193 CCND2
16 1/39 0.02770808 INHBB
17 1/47 0.02992601 INHBB
18 1/52 0.02992601 INHBB
19 1/54 0.02992601 CCND2
20 1/58 0.02992601 INHBB
21 1/58 0.02992601 INHBB
22 1/58 0.02992601 CCND2
23 1/66 0.03119720 INHBB
24 1/66 0.03119720 CCND2
25 1/71 0.03220612 CCND2
26 1/78 0.03397975 GTF2I
27 1/82 0.03397975 CCND2
28 1/84 0.03397975 INHBB
29 1/87 0.03397975 GTF2I
30 1/92 0.03472183 INHBB
31 1/104 0.03633053 INHBB
32 1/106 0.03633053 CCND2
33 1/106 0.03633053 INHBB
34 1/111 0.03691143 CCND2
35 1/120 0.03873796 ANK1
36 1/129 0.04038846 INHBB
37 1/133 0.04038846 INHBB
38 1/136 0.04038846 ANK1
39 1/140 0.04049813 ANK1
40 1/158 0.04450223 INHBB
41 1/182 0.04952524 INHBB
42 1/185 0.04952524 ANK1
[1] "GO_Cellular_Component_2021"
Term Overlap
1 spectrin-associated cytoskeleton (GO:0014731) 1/8
2 cyclin-dependent protein kinase holoenzyme complex (GO:0000307) 1/30
3 serine/threonine protein kinase complex (GO:1902554) 1/37
4 cytoplasmic side of plasma membrane (GO:0009898) 1/55
5 intracellular non-membrane-bounded organelle (GO:0043232) 2/1158
Adjusted.P.value Genes
1 0.02078867 ANK1
2 0.03197981 CCND2
3 0.03197981 CCND2
4 0.03560511 ANK1
5 0.04831435 CCND2;ANK1
[1] "GO_Molecular_Function_2021"
[1] Term Overlap Adjusted.P.value Genes
<0 rows> (or 0-length row.names)
Description
38 Thymic epithelial tumor
50 SPHEROCYTOSIS, TYPE 1 (disorder)
56 MEGALENCEPHALY-POLYMICROGYRIA-POLYDACTYLY-HYDROCEPHALUS SYNDROME 3
58 8p11.2 deletion syndrome
17 Acute Myeloid Leukemia, M1
31 Familial Testotoxicosis
46 POSTAXIAL POLYDACTYLY, TYPE B
47 Acute Myeloid Leukemia (AML-M2)
48 Alcohol Toxicity
55 MEGALENCEPHALY-POLYMICROGYRIA-POLYDACTYLY-HYDROCEPHALUS SYNDROME 1
FDR Ratio BgRatio
38 0.005978149 1/4 1/9703
50 0.005978149 1/4 1/9703
56 0.005978149 1/4 1/9703
58 0.005978149 1/4 1/9703
17 0.006519600 2/4 125/9703
31 0.006519600 1/4 3/9703
46 0.006519600 1/4 3/9703
47 0.006519600 2/4 125/9703
48 0.006519600 1/4 2/9703
55 0.006519600 1/4 3/9703******************************************
* *
* Welcome to WebGestaltR ! *
* *
******************************************Loading the functional categories...
Loading the ID list...
Loading the reference list...
Performing the enrichment analysis...Warning in oraEnrichment(interestGeneList, referenceGeneList, geneSet,
minNum = minNum, : No significant gene set is identified based on FDR 0.05!NULLSensitivity, specificity and precision for silver standard genes
library("readxl")
known_annotations <- read_xlsx("data/summary_known_genes_annotations.xlsx", sheet="T2D")
known_annotations <- unique(known_annotations$`Gene Symbol`)
unrelated_genes <- ctwas_gene_res$genename[!(ctwas_gene_res$genename %in% known_annotations)]
#number of genes in known annotations
print(length(known_annotations))[1] 72#number of genes in known annotations with imputed expression
print(sum(known_annotations %in% ctwas_gene_res$genename))[1] 35#assign ctwas, TWAS, and bystander genes
ctwas_genes <- ctwas_gene_res$genename[ctwas_gene_res$susie_pip>0.8]
twas_genes <- ctwas_gene_res$genename[abs(ctwas_gene_res$z)>sig_thresh]
novel_genes <- ctwas_genes[!(ctwas_genes %in% twas_genes)]
#significance threshold for TWAS
print(sig_thresh)[1] 4.609947#number of ctwas genes
length(ctwas_genes)[1] 4#number of TWAS genes
length(twas_genes)[1] 39#show novel genes (ctwas genes with not in TWAS genes)
ctwas_gene_res[ctwas_gene_res$genename %in% novel_genes,report_cols] genename region_tag susie_pip mu2 PVE z
7923 INHBB 2_70 0.902 22.2 5.9e-05 -4.47#sensitivity / recall
sensitivity <- rep(NA,2)
names(sensitivity) <- c("ctwas", "TWAS")
sensitivity["ctwas"] <- sum(ctwas_genes %in% known_annotations)/length(known_annotations)
sensitivity["TWAS"] <- sum(twas_genes %in% known_annotations)/length(known_annotations)
sensitivity ctwas TWAS
0.00000000 0.02777778 #specificity
specificity <- rep(NA,2)
names(specificity) <- c("ctwas", "TWAS")
specificity["ctwas"] <- sum(!(unrelated_genes %in% ctwas_genes))/length(unrelated_genes)
specificity["TWAS"] <- sum(!(unrelated_genes %in% twas_genes))/length(unrelated_genes)
specificity ctwas TWAS
0.9996769 0.9970111 #precision / PPV
precision <- rep(NA,2)
names(precision) <- c("ctwas", "TWAS")
precision["ctwas"] <- sum(ctwas_genes %in% known_annotations)/length(ctwas_genes)
precision["TWAS"] <- sum(twas_genes %in% known_annotations)/length(twas_genes)
precision ctwas TWAS
0.00000000 0.05128205 #ROC curves
pip_range <- (0:1000)/1000
sensitivity <- rep(NA, length(pip_range))
specificity <- rep(NA, length(pip_range))
for (index in 1:length(pip_range)){
pip <- pip_range[index]
ctwas_genes <- ctwas_gene_res$genename[ctwas_gene_res$susie_pip>=pip]
sensitivity[index] <- sum(ctwas_genes %in% known_annotations)/length(known_annotations)
specificity[index] <- sum(!(unrelated_genes %in% ctwas_genes))/length(unrelated_genes)
}
plot(1-specificity, sensitivity, type="l", xlim=c(0,1), ylim=c(0,1))
sig_thresh_range <- seq(from=0, to=max(abs(ctwas_gene_res$z)), length.out=length(pip_range))
for (index in 1:length(sig_thresh_range)){
sig_thresh_plot <- sig_thresh_range[index]
twas_genes <- ctwas_gene_res$genename[abs(ctwas_gene_res$z)>=sig_thresh_plot]
sensitivity[index] <- sum(twas_genes %in% known_annotations)/length(known_annotations)
specificity[index] <- sum(!(unrelated_genes %in% twas_genes))/length(unrelated_genes)
}
lines(1-specificity, sensitivity, xlim=c(0,1), ylim=c(0,1), col="red", lty=2)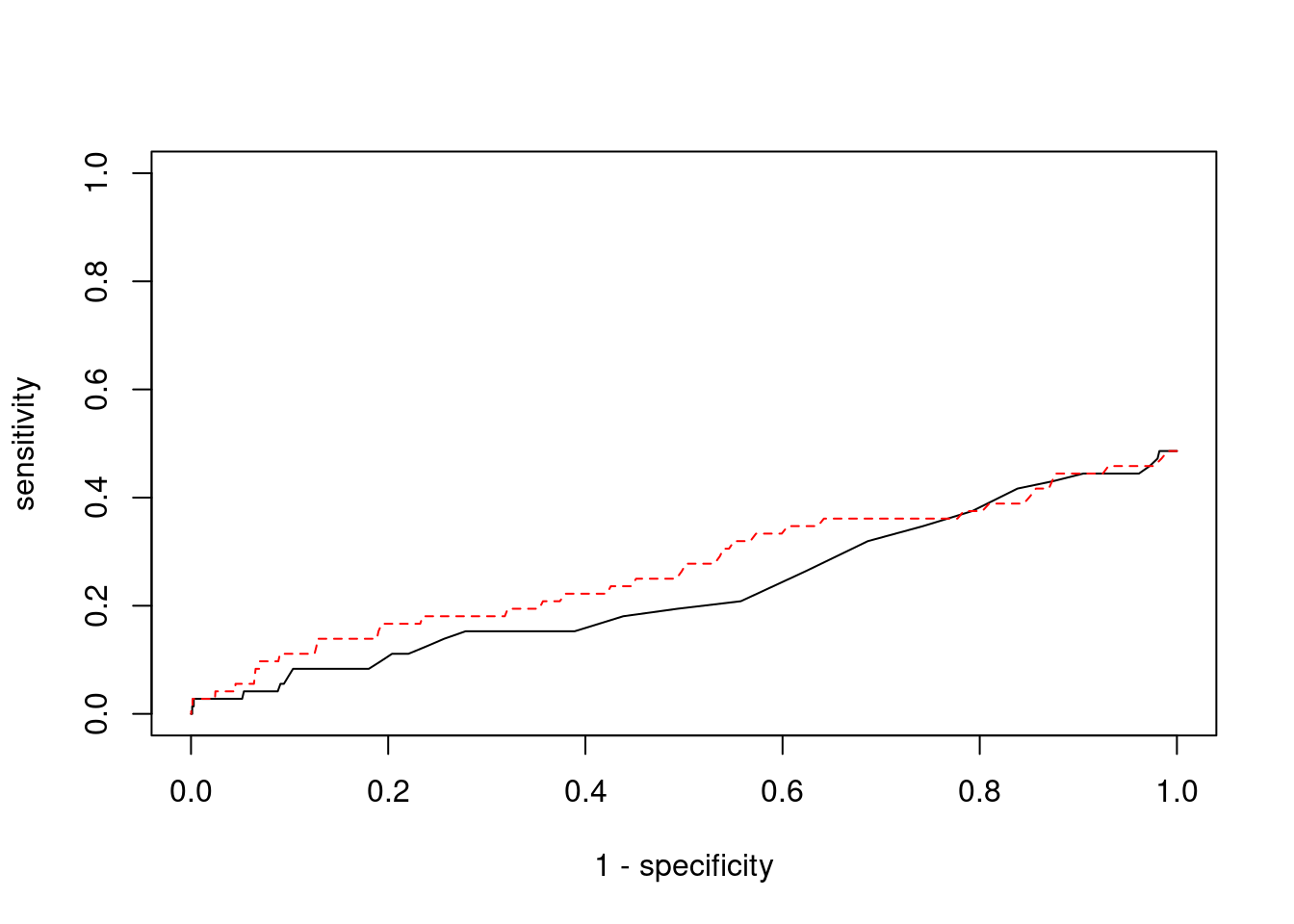
| Version | Author | Date |
|---|---|---|
| 3a7fbc1 | wesleycrouse | 2021-09-08 |
Sensitivity, specificity and precision for silver standard genes - bystanders only
This section first uses all silver standard genes to identify bystander genes within 1Mb. The silver standard and bystander gene lists are then subset to only genes with imputed expression in this analysis. Then, the ctwas and TWAS gene lists from this analysis are subset to only genes that are in the (subset) silver standard and bystander genes. These gene lists are then used to compute sensitivity, specificity and precision for ctwas and TWAS.
library(biomaRt)
library(GenomicRanges)Loading required package: stats4Loading required package: BiocGenericsLoading required package: parallel
Attaching package: 'BiocGenerics'The following objects are masked from 'package:parallel':
clusterApply, clusterApplyLB, clusterCall, clusterEvalQ,
clusterExport, clusterMap, parApply, parCapply, parLapply,
parLapplyLB, parRapply, parSapply, parSapplyLBThe following objects are masked from 'package:stats':
IQR, mad, sd, var, xtabsThe following objects are masked from 'package:base':
anyDuplicated, append, as.data.frame, basename, cbind,
colnames, dirname, do.call, duplicated, eval, evalq, Filter,
Find, get, grep, grepl, intersect, is.unsorted, lapply, Map,
mapply, match, mget, order, paste, pmax, pmax.int, pmin,
pmin.int, Position, rank, rbind, Reduce, rownames, sapply,
setdiff, sort, table, tapply, union, unique, unsplit, which,
which.max, which.minLoading required package: S4Vectors
Attaching package: 'S4Vectors'The following object is masked from 'package:base':
expand.gridLoading required package: IRangesLoading required package: GenomeInfoDbensembl <- useEnsembl(biomart="ENSEMBL_MART_ENSEMBL", dataset="hsapiens_gene_ensembl")
G_list <- getBM(filters= "chromosome_name", attributes= c("hgnc_symbol","chromosome_name","start_position","end_position","gene_biotype"), values=1:22, mart=ensembl)
G_list <- G_list[G_list$hgnc_symbol!="",]
G_list <- G_list[G_list$gene_biotype %in% c("protein_coding","lncRNA"),]
G_list$start <- G_list$start_position
G_list$end <- G_list$end_position
G_list_granges <- makeGRangesFromDataFrame(G_list, keep.extra.columns=T)
known_annotations_positions <- G_list[G_list$hgnc_symbol %in% known_annotations,]
half_window <- 1000000
known_annotations_positions$start <- known_annotations_positions$start_position - half_window
known_annotations_positions$end <- known_annotations_positions$end_position + half_window
known_annotations_positions$start[known_annotations_positions$start<1] <- 1
known_annotations_granges <- makeGRangesFromDataFrame(known_annotations_positions, keep.extra.columns=T)
bystanders <- findOverlaps(known_annotations_granges,G_list_granges)
bystanders <- unique(subjectHits(bystanders))
bystanders <- G_list$hgnc_symbol[bystanders]
bystanders <- bystanders[!(bystanders %in% known_annotations)]
unrelated_genes <- bystanders
#number of genes in known annotations
print(length(known_annotations))[1] 72#number of genes in known annotations with imputed expression
print(sum(known_annotations %in% ctwas_gene_res$genename))[1] 35#number of bystander genes
print(length(unrelated_genes))[1] 1837#number of bystander genes with imputed expression
print(sum(unrelated_genes %in% ctwas_gene_res$genename))[1] 941#remove genes without imputed expression from gene lists
known_annotations <- known_annotations[known_annotations %in% ctwas_gene_res$genename]
unrelated_genes <- unrelated_genes[unrelated_genes %in% ctwas_gene_res$genename]
#assign ctwas and TWAS genes
ctwas_genes <- ctwas_gene_res$genename[ctwas_gene_res$susie_pip>0.8]
twas_genes <- ctwas_gene_res$genename[abs(ctwas_gene_res$z)>sig_thresh]
#significance threshold for TWAS
print(sig_thresh)[1] 4.609947#number of ctwas genes
length(ctwas_genes)[1] 4#number of ctwas genes in known annotations or bystanders
sum(ctwas_genes %in% c(known_annotations, unrelated_genes))[1] 0#number of ctwas genes
length(twas_genes)[1] 39#number of TWAS genes
sum(twas_genes %in% c(known_annotations, unrelated_genes))[1] 5#remove genes not in known or bystander lists from results
ctwas_genes <- ctwas_genes[ctwas_genes %in% c(known_annotations, unrelated_genes)]
twas_genes <- twas_genes[twas_genes %in% c(known_annotations, unrelated_genes)]
#sensitivity / recall
sensitivity <- rep(NA,2)
names(sensitivity) <- c("ctwas", "TWAS")
sensitivity["ctwas"] <- sum(ctwas_genes %in% known_annotations)/length(known_annotations)
sensitivity["TWAS"] <- sum(twas_genes %in% known_annotations)/length(known_annotations)
sensitivity ctwas TWAS
0.00000000 0.05714286 #specificity
specificity <- rep(NA,2)
names(specificity) <- c("ctwas", "TWAS")
specificity["ctwas"] <- sum(!(unrelated_genes %in% ctwas_genes))/length(unrelated_genes)
specificity["TWAS"] <- sum(!(unrelated_genes %in% twas_genes))/length(unrelated_genes)
specificity ctwas TWAS
1.0000000 0.9968119 #precision / PPV
precision <- rep(NA,2)
names(precision) <- c("ctwas", "TWAS")
precision["ctwas"] <- sum(ctwas_genes %in% known_annotations)/length(ctwas_genes)
precision["TWAS"] <- sum(twas_genes %in% known_annotations)/length(twas_genes)
precisionctwas TWAS
NaN 0.4
sessionInfo()R version 3.6.1 (2019-07-05)
Platform: x86_64-pc-linux-gnu (64-bit)
Running under: Scientific Linux 7.4 (Nitrogen)
Matrix products: default
BLAS/LAPACK: /software/openblas-0.2.19-el7-x86_64/lib/libopenblas_haswellp-r0.2.19.so
locale:
[1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C
[3] LC_TIME=en_US.UTF-8 LC_COLLATE=en_US.UTF-8
[5] LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
[7] LC_PAPER=en_US.UTF-8 LC_NAME=C
[9] LC_ADDRESS=C LC_TELEPHONE=C
[11] LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
attached base packages:
[1] parallel stats4 stats graphics grDevices utils datasets
[8] methods base
other attached packages:
[1] GenomicRanges_1.36.0 GenomeInfoDb_1.20.0 IRanges_2.18.1
[4] S4Vectors_0.22.1 BiocGenerics_0.30.0 biomaRt_2.40.1
[7] readxl_1.3.1 WebGestaltR_0.4.4 disgenet2r_0.99.2
[10] enrichR_3.0 cowplot_1.0.0 ggplot2_3.3.3
loaded via a namespace (and not attached):
[1] bitops_1.0-6 fs_1.3.1 bit64_4.0.5
[4] doParallel_1.0.16 progress_1.2.2 httr_1.4.1
[7] rprojroot_2.0.2 tools_3.6.1 doRNG_1.8.2
[10] utf8_1.2.1 R6_2.5.0 DBI_1.1.1
[13] colorspace_1.4-1 withr_2.4.1 tidyselect_1.1.0
[16] prettyunits_1.0.2 bit_4.0.4 curl_3.3
[19] compiler_3.6.1 git2r_0.26.1 Biobase_2.44.0
[22] labeling_0.3 scales_1.1.0 readr_1.4.0
[25] stringr_1.4.0 apcluster_1.4.8 digest_0.6.20
[28] rmarkdown_1.13 svglite_1.2.2 XVector_0.24.0
[31] pkgconfig_2.0.3 htmltools_0.3.6 fastmap_1.1.0
[34] rlang_0.4.11 RSQLite_2.2.7 farver_2.1.0
[37] generics_0.0.2 jsonlite_1.6 dplyr_1.0.7
[40] RCurl_1.98-1.1 magrittr_2.0.1 GenomeInfoDbData_1.2.1
[43] Matrix_1.2-18 Rcpp_1.0.6 munsell_0.5.0
[46] fansi_0.5.0 gdtools_0.1.9 lifecycle_1.0.0
[49] stringi_1.4.3 whisker_0.3-2 yaml_2.2.0
[52] zlibbioc_1.30.0 plyr_1.8.4 grid_3.6.1
[55] blob_1.2.1 promises_1.0.1 crayon_1.4.1
[58] lattice_0.20-38 hms_1.1.0 knitr_1.23
[61] pillar_1.6.1 igraph_1.2.4.1 rjson_0.2.20
[64] rngtools_1.5 reshape2_1.4.3 codetools_0.2-16
[67] XML_3.98-1.20 glue_1.4.2 evaluate_0.14
[70] data.table_1.14.0 vctrs_0.3.8 httpuv_1.5.1
[73] foreach_1.5.1 cellranger_1.1.0 gtable_0.3.0
[76] purrr_0.3.4 assertthat_0.2.1 cachem_1.0.5
[79] xfun_0.8 later_0.8.0 tibble_3.1.2
[82] iterators_1.0.13 AnnotationDbi_1.46.0 memoise_2.0.0
[85] workflowr_1.6.2 ellipsis_0.3.2